Structure of the human TRPM4 ion channel in a lipid nanodisc
- PMID: 29217581
- PMCID: PMC5898196
- DOI: 10.1126/science.aar4510
Structure of the human TRPM4 ion channel in a lipid nanodisc
Abstract
Transient receptor potential (TRP) melastatin 4 (TRPM4) is a widely expressed cation channel associated with a variety of cardiovascular disorders. TRPM4 is activated by increased intracellular calcium in a voltage-dependent manner but, unlike many other TRP channels, is permeable to monovalent cations only. Here we present two structures of full-length human TRPM4 embedded in lipid nanodiscs at ~3-angstrom resolution, as determined by single-particle cryo-electron microscopy. These structures, with and without calcium bound, reveal a general architecture for this major subfamily of TRP channels and a well-defined calcium-binding site within the intracellular side of the S1-S4 domain. The structures correspond to two distinct closed states. Calcium binding induces conformational changes that likely prime the channel for voltage-dependent opening.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

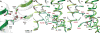
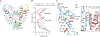
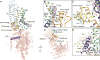
Comment in
-
TRPM channels come into focus.Science. 2018 Jan 12;359(6372):160-161. doi: 10.1126/science.aar6205. Science. 2018. PMID: 29326261 No abstract available.
Similar articles
-
Structures of the calcium-activated, non-selective cation channel TRPM4.Nature. 2017 Dec 14;552(7684):205-209. doi: 10.1038/nature24997. Epub 2017 Dec 6. Nature. 2017. PMID: 29211714 Free PMC article.
-
Structure of full-length human TRPM4.Proc Natl Acad Sci U S A. 2018 Mar 6;115(10):2377-2382. doi: 10.1073/pnas.1722038115. Epub 2018 Feb 20. Proc Natl Acad Sci U S A. 2018. PMID: 29463718 Free PMC article.
-
Electron cryo-microscopy structure of a human TRPM4 channel.Nature. 2017 Dec 14;552(7684):200-204. doi: 10.1038/nature24674. Epub 2017 Dec 6. Nature. 2017. PMID: 29211723
-
Regulation of TRP channels: a voltage-lipid connection.Biochem Soc Trans. 2007 Feb;35(Pt 1):105-8. doi: 10.1042/BST0350105. Biochem Soc Trans. 2007. PMID: 17233613 Review.
-
Determining the Crystal Structure of TRPV6.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. PMID: 30299652 Free Books & Documents. Review.
Cited by
-
Analysis of discrete local variability and structural covariance in macromolecular assemblies using Cryo-EM and focused classification.Ultramicroscopy. 2019 Aug;203:170-180. doi: 10.1016/j.ultramic.2018.11.016. Epub 2018 Nov 29. Ultramicroscopy. 2019. PMID: 30528101 Free PMC article.
-
Solubilization, purification, and ligand binding characterization of G protein-coupled receptor SMO in native membrane bilayer using styrene maleic acid copolymer.PeerJ. 2022 May 3;10:e13381. doi: 10.7717/peerj.13381. eCollection 2022. PeerJ. 2022. PMID: 35529497 Free PMC article.
-
Structure-function analyses of the ion channel TRPC3 reveal that its cytoplasmic domain allosterically modulates channel gating.J Biol Chem. 2018 Oct 12;293(41):16102-16114. doi: 10.1074/jbc.RA118.005066. Epub 2018 Aug 23. J Biol Chem. 2018. PMID: 30139744 Free PMC article.
-
A Systemic Review of the Integral Role of TRPM2 in Ischemic Stroke: From Upstream Risk Factors to Ultimate Neuronal Death.Cells. 2022 Jan 31;11(3):491. doi: 10.3390/cells11030491. Cells. 2022. PMID: 35159300 Free PMC article.
-
PIRT the TRP Channel Regulating Protein Binds Calmodulin and Cholesterol-Like Ligands.Biomolecules. 2020 Mar 21;10(3):478. doi: 10.3390/biom10030478. Biomolecules. 2020. PMID: 32245175 Free PMC article.
References
-
- Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. - PubMed
-
- Launay P, et al. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. - PubMed
-
- Launay P, et al. TRPM4 regulates calcium oscillations after T cell activation. Science. 2004;306:1374–1377. - PubMed
-
- Nilius B, et al. Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem. 2003;278:30813–30820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

