Cancer Energy Metabolism: Shutting Power off Cancer Factory
- PMID: 29212305
- PMCID: PMC5746036
- DOI: 10.4062/biomolther.2017.184
Cancer Energy Metabolism: Shutting Power off Cancer Factory
Abstract
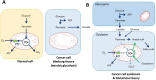
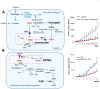
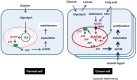
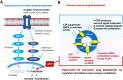
In 1923, Dr. Warburg had observed that tumors acidified the Ringer solution when 13 mM glucose was added, which was identified as being due to lactate. When glucose is the only source of nutrient, it can serve for both biosynthesis and energy production. However, a series of studies revealed that the cancer cell consumes glucose for biosynthesis through fermentation, not for energy supply, under physiological conditions. Recently, a new observation was made that there is a metabolic symbiosis in which glycolytic and oxidative tumor cells mutually regulate their energy metabolism. Hypoxic cancer cells use glucose for glycolytic metabolism and release lactate which is used by oxygenated cancer cells. This study challenged the Warburg effect, because Warburg claimed that fermentation by irreversible damaging of mitochondria is a fundamental cause of cancer. However, recent studies revealed that mitochondria in cancer cell show active function of oxidative phosphorylation although TCA cycle is stalled. It was also shown that blocking cytosolic NADH production by aldehyde dehydrogenase inhibition, combined with oxidative phosphorylation inhibition, resulted in up to 80% decrease of ATP production, which resulted in a significant regression of tumor growth in the NSCLC model. This suggests a new theory that NADH production in the cytosol plays a key role of ATP production through the mitochondrial electron transport chain in cancer cells, while NADH production is mostly occupied inside mitochondria in normal cells.
Keywords: Cancer energy metabolism; Electron transport chain; Malate-aspartate shuttle; Oxidative phosphorylation; TCA cycle; Warburg effect.
Figures
Similar articles
-
Subcellular metabolite transport and carbon isotope kinetics in the intramyocardial glutamate pool.Biochemistry. 1996 May 28;35(21):6963-8. doi: 10.1021/bi960199l. Biochemistry. 1996. PMID: 8639648
-
Loss of SLC25A11 causes suppression of NSCLC and melanoma tumor formation.EBioMedicine. 2019 Feb;40:184-197. doi: 10.1016/j.ebiom.2019.01.036. Epub 2019 Jan 25. EBioMedicine. 2019. PMID: 30686754 Free PMC article.
-
Separation of metabolic supply and demand: aerobic glycolysis as a normal physiological response to fluctuating energetic demands in the membrane.Cancer Metab. 2014 Jun 5;2:7. doi: 10.1186/2049-3002-2-7. eCollection 2014. Cancer Metab. 2014. PMID: 24982758 Free PMC article.
-
Importance of Michaelis Constants for Cancer Cell Redox Balance and Lactate Secretion-Revisiting the Warburg Effect.Cancers (Basel). 2024 Jun 21;16(13):2290. doi: 10.3390/cancers16132290. Cancers (Basel). 2024. PMID: 39001354 Free PMC article. Review.
-
Mitochondria and diabetes. Genetic, biochemical, and clinical implications of the cellular energy circuit.Diabetes. 1996 Feb;45(2):113-26. doi: 10.2337/diab.45.2.113. Diabetes. 1996. PMID: 8549853 Review.
Cited by
-
Targeting oxidative phosphorylation as an approach for the treatment of ovarian cancer.Front Oncol. 2022 Sep 6;12:971479. doi: 10.3389/fonc.2022.971479. eCollection 2022. Front Oncol. 2022. PMID: 36147929 Free PMC article. Review.
-
[NDRG2 inhibits tumorigenesis of hepatocellular carcinoma by regulating metabolism of phospholipids and triglyceride: a metabonomic analysis].Nan Fang Yi Ke Da Xue Xue Bao. 2022 Dec 20;42(12):1765-1773. doi: 10.12122/j.issn.1673-4254.2022.12.03. Nan Fang Yi Ke Da Xue Xue Bao. 2022. PMID: 36651243 Free PMC article. Chinese.
-
Pathway Analysis of Fucoidan Activity Using a Yeast Gene Deletion Library Screen.Mar Drugs. 2019 Jan 14;17(1):54. doi: 10.3390/md17010054. Mar Drugs. 2019. PMID: 30646537 Free PMC article.
-
Post-transcriptional diversity in riboproteins and RNAs in aging and cancer.Semin Cancer Biol. 2021 Nov;76:292-300. doi: 10.1016/j.semcancer.2021.08.012. Epub 2021 Aug 30. Semin Cancer Biol. 2021. PMID: 34474152 Free PMC article. Review.
-
Cancer Metabolism: a Hope for Curing Cancer.Biomol Ther (Seoul). 2018 Jan 1;26(1):1-3. doi: 10.4062/biomolther.2017.300. Biomol Ther (Seoul). 2018. PMID: 29212300 Free PMC article. No abstract available.
References
-
- Cheong JH, Park ES, Liang J, Dennison JB, Tsavachidou D, Nguyen-Charles C, Wa Cheng K, Hall H, Zhang D, Lu Y, Ravoori M, Kundra V, Ajani J, Lee JS, Ki Hong W, Mills GB. Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol Cancer Ther. 2011;10:2350–2362. doi: 10.1158/1535-7163.MCT-11-0497. - DOI - PMC - PubMed
-
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

