Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice
- PMID: 29192190
- PMCID: PMC5709495
- DOI: 10.1038/s41467-017-01739-2
Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice
Abstract
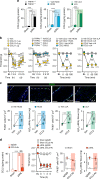
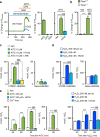
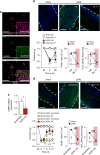
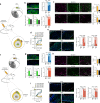
It is known that transient receptor potential ankyrin 1 (TRPA1) channels, expressed by nociceptors, contribute to neuropathic pain. Here we show that TRPA1 is also expressed in Schwann cells. We found that in mice with partial sciatic nerve ligation, TRPA1 silencing in nociceptors attenuated mechanical allodynia, without affecting macrophage infiltration and oxidative stress, whereas TRPA1 silencing in Schwann cells reduced both allodynia and neuroinflammation. Activation of Schwann cell TRPA1 evoked NADPH oxidase 1 (NOX1)-dependent H2O2 release, and silencing or blocking Schwann cell NOX1 attenuated nerve injury-induced macrophage infiltration, oxidative stress and allodynia. Furthermore, the NOX2-dependent oxidative burst, produced by macrophages recruited to the perineural space activated the TRPA1-NOX1 pathway in Schwann cells, but not TRPA1 in nociceptors. Schwann cell TRPA1 generates a spatially constrained gradient of oxidative stress, which maintains macrophage infiltration to the injured nerve, and sends paracrine signals to activate TRPA1 of ensheathed nociceptors to sustain mechanical allodynia.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Schwann cells expressing nociceptive channel TRPA1 orchestrate ethanol-evoked neuropathic pain in mice.J Clin Invest. 2019 Dec 2;129(12):5424-5441. doi: 10.1172/JCI128022. J Clin Invest. 2019. PMID: 31487269 Free PMC article.
-
Schwann cell TRPA1 elicits reserpine-induced fibromyalgia pain in mice.Br J Pharmacol. 2024 Sep;181(18):3445-3461. doi: 10.1111/bph.16413. Epub 2024 May 21. Br J Pharmacol. 2024. PMID: 38772415
-
Macrophages and Schwann cell TRPA1 mediate chronic allodynia in a mouse model of complex regional pain syndrome type I.Brain Behav Immun. 2020 Aug;88:535-546. doi: 10.1016/j.bbi.2020.04.037. Epub 2020 Apr 18. Brain Behav Immun. 2020. PMID: 32315759
-
Neuronal and non-neuronal TRPA1 as therapeutic targets for pain and headache relief.Temperature (Austin). 2022 May 29;10(1):50-66. doi: 10.1080/23328940.2022.2075218. eCollection 2023. Temperature (Austin). 2022. PMID: 37187829 Free PMC article. Review.
-
TRPA1 as a therapeutic target for nociceptive pain.Expert Opin Ther Targets. 2020 Oct;24(10):997-1008. doi: 10.1080/14728222.2020.1815191. Epub 2020 Sep 11. Expert Opin Ther Targets. 2020. PMID: 32838583 Free PMC article. Review.
Cited by
-
Novel Cinnamaldehyde Derivatives Inhibit Peripheral Nerve Degeneration by Targeting Schwann Cells.Antioxidants (Basel). 2022 Sep 20;11(10):1846. doi: 10.3390/antiox11101846. Antioxidants (Basel). 2022. PMID: 36290569 Free PMC article.
-
Schwann cells expressing nociceptive channel TRPA1 orchestrate ethanol-evoked neuropathic pain in mice.J Clin Invest. 2019 Dec 2;129(12):5424-5441. doi: 10.1172/JCI128022. J Clin Invest. 2019. PMID: 31487269 Free PMC article.
-
Effects of NADPH Oxidase Isoform-2 (NOX2) Inhibition on Behavioral Responses and Neuroinflammation in a Mouse Model of Neuropathic Pain.Biomedicines. 2023 Jan 31;11(2):416. doi: 10.3390/biomedicines11020416. Biomedicines. 2023. PMID: 36830952 Free PMC article.
-
Piezo channels contribute to the regulation of myelination in Schwann cells.Glia. 2022 Dec;70(12):2276-2289. doi: 10.1002/glia.24251. Epub 2022 Jul 29. Glia. 2022. PMID: 35903933 Free PMC article.
-
Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain.Nat Commun. 2020 Jan 14;11(1):264. doi: 10.1038/s41467-019-13839-2. Nat Commun. 2020. PMID: 31937758 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

