Pneumococcal conjugate vaccine 13 delivered as one primary and one booster dose (1 + 1) compared with two primary doses and a booster (2 + 1) in UK infants: a multicentre, parallel group randomised controlled trial
- PMID: 29174323
- PMCID: PMC5805912
- DOI: 10.1016/S1473-3099(17)30654-0
Pneumococcal conjugate vaccine 13 delivered as one primary and one booster dose (1 + 1) compared with two primary doses and a booster (2 + 1) in UK infants: a multicentre, parallel group randomised controlled trial
Abstract
Background: Infants in the UK were first offered a pneumococcal conjugate vaccine (PCV7) in 2006, given at 2 and 4 months of age and a booster dose at 13 months (2 + 1 schedule). A 13-valent vaccine (PCV13) replaced PCV7 in 2010. We aimed to compare the post-booster antibody response in UK infants given a reduced priming schedule of PCV13 (ie, a 1 + 1 schedule) versus the current 2 + 1 schedule and to assess the potential effect on population protection.
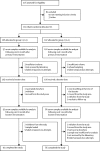
Methods: In this multicentre, parallel group, randomised controlled trial, we recuited infants due to receive their primary immunisations aged up to 13 weeks on first vaccinations by information booklets mailed out via the NHS Child Health Information Service and the UK National Health Application and Infrastructure Services. Eligible infants were randomly assigned (1:1) to receive PCV13 at 2, 4, and 12 months (2 + 1 schedule) or 3 and 12 months of age (1 + 1 schedule) delivered with other routine vaccinations. Randomisation was done by computer-generated permuted block randomisation, with a block size of six. Participants and clinical trial staff were not masked to treatment allocation. The primary endpoint was serotype-specific immunoglobulin G concentrations values (geometric mean concentrations [GMC] in μg/mL) measured in blood samples collected at 13 months of age. Analysis was by modified intention to treat with all individuals included by randomised group if they had a laboratory result. This trial is registered on the EudraCT clinical trial database, number 2015-000817-32, and ClinicalTrials.gov, number NCT02482636.
Findings: Between September, 2015, and June, 2016, 376 infants were assessed for eligibility. 81 infants were excluded for not meeting the inclusion criteria (n=50) or for other reasons (n=31). 213 eligible infants were enrolled and randomly allocated to group 1 (n=106; 2 + 1 schedule) or to group 2 (n=107; 1 + 1 schedule). In group 1, 91 serum samples were available for analysis 1 month after booster immunisation versus 86 in group 2. At month 13, post-booster, GMCs were equivalent between schedules for serotypes 3 (0·61 μg/mL in group 1 vs 0·62 μg/mL in group 2), 5 (1·74 μg/mL vs 2·11 μg/mL), 7F (3·98 μg/mL vs 3·36 μg/mL), 9V (2·34 μg/mL vs 2·50 μg/mL), and 19A (8·38 μg/mL vs 8·83 μg/mL). Infants given the 1 + 1 schedule had significantly greater immunogenicity post-booster than those given the 2 + 1 schedule for serotypes 1 (8·92 μg/mL vs 3·07 μg/mL), 4 (3·43 μg/mL vs 2·55 μg/mL), 14 (16·9 μg/mL vs 10·49 μg/mL), and 19F (14·76 μg/mL vs 11·12 μg/mL; adjusted p value range <0·001 to 0·047). The 2 + 1 schedule was superior for serotypes 6A, 6B, 18C and 23F (adjusted p value range <0·0001 to 0·017). In a predefined numerical subset of all of the infants recruited to the study (n=40 [20%]), functional serotype-specific antibody was similar between schedules. 26 serious adverse events were recorded in 21 (10%) infants across the study period; 18 (n=13) were in the 2 + 1 group and eight (n=8) in the 1 + 1 group. Only one serious adverse event, a high temperature and refusal to feed after the first vaccination visit in a child on the 2+1 schedule was considered related to vaccine.
Interpretation: Our findings show that for nine of the 13 serotypes in PCV13, post-booster responses in infants primed with a single dose are equivalent or superior to those seen following the standard UK 2 + 1 schedule. Introducing a 1 + 1 schedule in countries with a mature PCV programme and established herd immunity is likely to maintain population control of vaccine-type pneumococcal disease.
Funding: NIHR and the Bill & Melinda Gates Foundation.
Copyright © 2018 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Comment in
-
When less is more: how many doses of PCV are enough?Lancet Infect Dis. 2018 Feb;18(2):127-128. doi: 10.1016/S1473-3099(17)30684-9. Epub 2017 Nov 22. Lancet Infect Dis. 2018. PMID: 29174324 No abstract available.
-
Reassessing the 1 + 1 pneumococcal conjugate vaccine schedule.Lancet Infect Dis. 2018 Apr;18(4):381-382. doi: 10.1016/S1473-3099(18)30129-4. Epub 2018 Mar 21. Lancet Infect Dis. 2018. PMID: 29582765 No abstract available.
-
Reassessing the 1 + 1 pneumococcal conjugate vaccine schedule - Authors' reply.Lancet Infect Dis. 2018 Apr;18(4):382-383. doi: 10.1016/S1473-3099(18)30168-3. Epub 2018 Mar 21. Lancet Infect Dis. 2018. PMID: 29582766 No abstract available.
-
Reassessing the 1 + 1 pneumococcal conjugate vaccine schedule.Lancet Infect Dis. 2018 Apr;18(4):382. doi: 10.1016/S1473-3099(18)30133-6. Epub 2018 Mar 21. Lancet Infect Dis. 2018. PMID: 29582767 No abstract available.
Similar articles
-
Immunogenicity and safety of a 10-valent pneumococcal conjugate vaccine administered as a 2 + 1 schedule to healthy infants in The Gambia: a single-centre, double-blind, active-controlled, randomised, phase 3 trial.Lancet Infect Dis. 2023 May;23(5):609-620. doi: 10.1016/S1473-3099(22)00734-4. Epub 2023 Jan 10. Lancet Infect Dis. 2023. PMID: 36638819 Clinical Trial.
-
Immunogenicity of a single-dose compared with a two-dose primary series followed by a booster dose of ten-valent or 13-valent pneumococcal conjugate vaccine in South African children: an open-label, randomised, non-inferiority trial.Lancet Infect Dis. 2020 Dec;20(12):1426-1436. doi: 10.1016/S1473-3099(20)30289-9. Epub 2020 Aug 25. Lancet Infect Dis. 2020. PMID: 32857992 Free PMC article. Clinical Trial.
-
Immunogenicity and safety of a novel ten-valent pneumococcal conjugate vaccine in healthy infants in The Gambia: a phase 3, randomised, double-blind, non-inferiority trial.Lancet Infect Dis. 2021 Jun;21(6):834-846. doi: 10.1016/S1473-3099(20)30735-0. Epub 2021 Jan 28. Lancet Infect Dis. 2021. PMID: 33516293 Clinical Trial.
-
Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on immunogenicity.Pediatr Infect Dis J. 2014 Jan;33 Suppl 2(Suppl 2 Optimum Dosing of Pneumococcal Conjugate Vaccine For Infants 0 A Landscape Analysis of Evidence Supportin g Different Schedules):S119-29. doi: 10.1097/INF.0000000000000079. Pediatr Infect Dis J. 2014. PMID: 24336054 Free PMC article. Review.
-
13-valent pneumococcal conjugate vaccine (PCV13).Hum Vaccin. 2011 Oct;7(10):1012-8. doi: 10.4161/hv.7.10.16794. Epub 2011 Oct 1. Hum Vaccin. 2011. PMID: 21941097 Review.
Cited by
-
Technological approaches to streamline vaccination schedules, progressing towards single-dose vaccines.NPJ Vaccines. 2020 Sep 18;5:88. doi: 10.1038/s41541-020-00238-8. eCollection 2020. NPJ Vaccines. 2020. PMID: 33024579 Free PMC article. Review.
-
Prevalence and serotype distribution of nasopharyngeal carriage of Streptococcus pneumoniae among healthy children under 5 years of age in Hainan Province, China.Infect Dis Poverty. 2024 Jan 19;13(1):7. doi: 10.1186/s40249-024-01175-7. Infect Dis Poverty. 2024. PMID: 38238873 Free PMC article.
-
Meeting report: Global vaccine and immunization research forum, 2018.Vaccine. 2019 Dec 10;37(52):7519-7526. doi: 10.1016/j.vaccine.2019.10.011. Epub 2019 Oct 14. Vaccine. 2019. PMID: 31623915 Free PMC article.
-
Vaccinology in sub-Saharan Africa.BMJ Glob Health. 2019 Sep 20;4(5):e001363. doi: 10.1136/bmjgh-2018-001363. eCollection 2019. BMJ Glob Health. 2019. PMID: 31637022 Free PMC article. Review.
-
Epidemiology of non-vaccine serotypes of Streptococcus pneumoniae before and after universal administration of pneumococcal conjugate vaccines.Hum Vaccin Immunother. 2021 Dec 2;17(12):5628-5637. doi: 10.1080/21645515.2021.1985353. Epub 2021 Nov 2. Hum Vaccin Immunother. 2021. PMID: 34726580 Free PMC article. Review.
References
-
- Whitney CG, Farley MM, Hadler J. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746. - PubMed
-
- Whitney CG, Pilishvili T, Farley MM. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368:1495–1502. - PubMed
-
- Goldblatt D, Southern J, Ashton L. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 2006;25:312–319. - PubMed
-
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–768. - PubMed
-
- Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15:535–543. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical