Cartilage intermediate layer protein 1 (CILP1): A novel mediator of cardiac extracellular matrix remodelling
- PMID: 29167509
- PMCID: PMC5700204
- DOI: 10.1038/s41598-017-16201-y
Cartilage intermediate layer protein 1 (CILP1): A novel mediator of cardiac extracellular matrix remodelling
Abstract
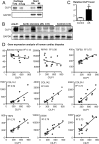
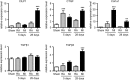
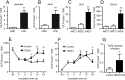
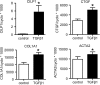
Heart failure is accompanied by extracellular matrix (ECM) remodelling, often leading to cardiac fibrosis. In the present study we explored the significance of cartilage intermediate layer protein 1 (CILP1) as a novel mediator of cardiac ECM remodelling. Whole genome transcriptional analysis of human cardiac tissue samples revealed a strong association of CILP1 with many structural (e.g. COL1A2 r2 = 0.83) and non-structural (e.g. TGFB3 r2 = 0.75) ECM proteins. Gene enrichment analysis further underscored the involvement of CILP1 in human cardiac ECM remodelling and TGFβ signalling. Myocardial CILP1 protein levels were significantly elevated in human infarct tissue and in aortic valve stenosis patients. CILP1 mRNA levels markedly increased in mouse heart after myocardial infarction, transverse aortic constriction, and angiotensin II treatment. Cardiac fibroblasts were found to be the primary source of cardiac CILP1 expression. Recombinant CILP1 inhibited TGFβ-induced αSMA gene and protein expression in cardiac fibroblasts. In addition, CILP1 overexpression in HEK293 cells strongly (5-fold p < 0.05) inhibited TGFβ signalling activity. In conclusion, our study identifies CILP1 as a new cardiac matricellular protein interfering with pro-fibrotic TGFβ signalling, and as a novel sensitive marker for cardiac fibrosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Cartilage intermediate layer protein-1 alleviates pressure overload-induced cardiac fibrosis via interfering TGF-β1 signaling.J Mol Cell Cardiol. 2018 Mar;116:135-144. doi: 10.1016/j.yjmcc.2018.02.006. Epub 2018 Feb 10. J Mol Cell Cardiol. 2018. PMID: 29438665
-
Endoplasmic Reticulum Protein TXNDC5 Augments Myocardial Fibrosis by Facilitating Extracellular Matrix Protein Folding and Redox-Sensitive Cardiac Fibroblast Activation.Circ Res. 2018 Apr 13;122(8):1052-1068. doi: 10.1161/CIRCRESAHA.117.312130. Epub 2018 Mar 13. Circ Res. 2018. PMID: 29535165 Free PMC article.
-
Matricellular Protein Cilp1 Promotes Myocardial Fibrosis in Response to Myocardial Infarction.Circ Res. 2021 Nov 12;129(11):1021-1035. doi: 10.1161/CIRCRESAHA.121.319482. Epub 2021 Oct 6. Circ Res. 2021. PMID: 34610755 Free PMC article.
-
Fibroblasts and the extracellular matrix in right ventricular disease.Cardiovasc Res. 2017 Oct 1;113(12):1453-1464. doi: 10.1093/cvr/cvx146. Cardiovasc Res. 2017. PMID: 28957531 Free PMC article. Review.
-
Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis.Cardiovasc Res. 2000 May;46(2):257-63. doi: 10.1016/s0008-6363(00)00030-4. Cardiovasc Res. 2000. PMID: 10773229 Review.
Cited by
-
Can cartilage intermediate layer protein 1 (CILP1) use as a novel biomarker for canine myxomatous mitral valve degeneration levels or not?BMC Vet Res. 2023 Mar 7;19(1):59. doi: 10.1186/s12917-023-03583-7. BMC Vet Res. 2023. PMID: 36882760 Free PMC article.
-
Integrated analysis of mRNA and microRNA expression profiles reveals differential transcriptome signature in ischaemic and dilated cardiomyopathy induced heart failure.Epigenetics. 2021 Aug;16(8):917-932. doi: 10.1080/15592294.2020.1827721. Epub 2020 Oct 4. Epigenetics. 2021. PMID: 33016206 Free PMC article.
-
Proteomics of CKD progression in the chronic renal insufficiency cohort.Nat Commun. 2023 Oct 10;14(1):6340. doi: 10.1038/s41467-023-41642-7. Nat Commun. 2023. PMID: 37816758 Free PMC article.
-
Cardiac Fibrosis Is Associated With Decreased Circulating Levels of Full-Length CILP in Heart Failure.JACC Basic Transl Sci. 2020 Apr 15;5(5):432-443. doi: 10.1016/j.jacbts.2020.01.016. eCollection 2020 May. JACC Basic Transl Sci. 2020. PMID: 32478206 Free PMC article.
-
scRNA-seq generates a molecular map of emerging cell subtypes after sciatic nerve injury in rats.Commun Biol. 2022 Oct 19;5(1):1105. doi: 10.1038/s42003-022-03970-0. Commun Biol. 2022. PMID: 36261573 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

