Granulocyte-derived TNFα promotes vascular and hematopoietic regeneration in the bone marrow
- PMID: 29155425
- PMCID: PMC5760474
- DOI: 10.1038/nm.4448
Granulocyte-derived TNFα promotes vascular and hematopoietic regeneration in the bone marrow
Abstract
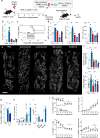
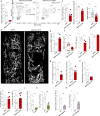
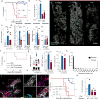
Endothelial cells are a critical component of the bone marrow (BM) stromal network, which maintains and regulates hematopoietic cells. Vascular regeneration precedes, and is necessary for, successful hematopoietic stem cell (HSC) transplantation, the only cure for most hematopoietic diseases. Recent data suggest that mature hematopoietic cells regulate BM stromal-cell function. Whether a similar cross-talk regulates the BM vasculature is not known. Here we found that donor hematopoietic cells act on sinusoidal endothelial cells and induce host blood vessel and hematopoietic regeneration after BM transplantation in mice. Adoptive transfer of BM, but not peripheral, granulocytes prevented the death of mice transplanted with limited numbers of HSCs and accelerated recovery of host vessels and hematopoietic cells. Moreover, selective granulocyte ablation in vivo impaired vascular and hematopoietic regeneration after BM transplantation. Gene expression analyses indicated that granulocytes are the main source of the cytokine TNFα, whereas its receptor TNFR1 is selectively upregulated in regenerating blood vessels. In adoptive transfer experiments, wild type, but not Tnfa-/-, granulocytes induced vascular recovery, and wild-type granulocyte transfer did not prevent death or promote vascular regeneration in Tnfr1-/-; Tnfr2-/- mice. Thus, by delivering TNFα to endothelial cells, granulocytes promote blood vessel growth and hematopoietic regeneration. Manipulation of the cross-talk between granulocytes and endothelial cells may lead to new therapeutic approaches to improve blood vessel regeneration and increase survival and hematopoietic recovery after HSC transplantation.
Figures

Similar articles
-
Evidence that a lipolytic enzyme--hematopoietic-specific phospholipase C-β2--promotes mobilization of hematopoietic stem cells by decreasing their lipid raft-mediated bone marrow retention and increasing the promobilizing effects of granulocytes.Leukemia. 2016 Apr;30(4):919-28. doi: 10.1038/leu.2015.315. Epub 2015 Nov 19. Leukemia. 2016. PMID: 26582648 Free PMC article.
-
Hematopoietic progenitor cell mobilization results in hypoxia with increased hypoxia-inducible transcription factor-1 alpha and vascular endothelial growth factor A in bone marrow.Stem Cells. 2007 Aug;25(8):1954-65. doi: 10.1634/stemcells.2006-0688. Epub 2007 May 3. Stem Cells. 2007. PMID: 17478585
-
Bone marrow angiotensin AT1 receptor regulates differentiation of monocyte lineage progenitors from hematopoietic stem cells.Arterioscler Thromb Vasc Biol. 2009 Oct;29(10):1529-36. doi: 10.1161/ATVBAHA.109.187732. Epub 2009 Jul 23. Arterioscler Thromb Vasc Biol. 2009. PMID: 19628784
-
Hematopoietic stem cells: potential new applications for translational medicine.J Stem Cells. 2014;9(3):163-97. J Stem Cells. 2014. PMID: 25157450 Review.
-
Implication for bone marrow derived stem cells in hepatocyte regeneration after orthotopic liver transplantation.Int J Hepatol. 2013;2013:310612. doi: 10.1155/2013/310612. Epub 2013 Sep 10. Int J Hepatol. 2013. PMID: 24109514 Free PMC article. Review.
Cited by
-
Cellular niches for hematopoietic stem cells in bone marrow under normal and malignant conditions.Inflamm Regen. 2023 Feb 21;43(1):15. doi: 10.1186/s41232-023-00267-5. Inflamm Regen. 2023. PMID: 36805714 Free PMC article. Review.
-
Targeting the Hematopoietic Stem Cell Niche in β-Thalassemia and Sickle Cell Disease.Pharmaceuticals (Basel). 2022 May 11;15(5):592. doi: 10.3390/ph15050592. Pharmaceuticals (Basel). 2022. PMID: 35631417 Free PMC article. Review.
-
miR-7977 inhibits the Hippo-YAP signaling pathway in bone marrow mesenchymal stromal cells.PLoS One. 2019 Mar 5;14(3):e0213220. doi: 10.1371/journal.pone.0213220. eCollection 2019. PLoS One. 2019. PMID: 30835743 Free PMC article.
-
Complement dependent TNFα production in neutrophil-like HL60 cells.Biochem Biophys Rep. 2023 Apr 14;34:101465. doi: 10.1016/j.bbrep.2023.101465. eCollection 2023 Jul. Biochem Biophys Rep. 2023. PMID: 37125077 Free PMC article.
-
Lactate released by inflammatory bone marrow neutrophils induces their mobilization via endothelial GPR81 signaling.Nat Commun. 2020 Jul 15;11(1):3547. doi: 10.1038/s41467-020-17402-2. Nat Commun. 2020. PMID: 32669546 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

