Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination
- PMID: 29142145
- PMCID: PMC5800335
- DOI: 10.1136/jnnp-2017-316880
Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination
Abstract
Objective: We characterised the clinical course, treatment and outcomes in 59 patients with relapsing myelin oligodendrocyte glycoprotein (MOG) antibody-associated demyelination.
Methods: We evaluated clinical phenotypes, annualised relapse rates (ARR) prior and on immunotherapy and Expanded Disability Status Scale (EDSS), in 218 demyelinating episodes from 33 paediatric and 26 adult patients.
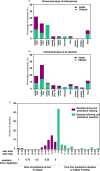
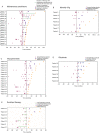
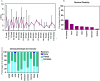
Results: The most common initial presentation in the cohort was optic neuritis (ON) in 54% (bilateral (BON) 32%, unilateral (UON) 22%), followed by acute disseminated encephalomyelitis (ADEM) (20%), which occurred exclusively in children. ON was the dominant phenotype (UON 35%, BON 19%) of all clinical episodes. 109/226 (48%) MRIs had no brain lesions. Patients were steroid responsive, but 70% of episodes treated with oral prednisone relapsed, particularly at doses <10 mg daily or within 2 months of cessation. Immunotherapy, including maintenance prednisone (P=0.0004), intravenous immunoglobulin, rituximab and mycophenolate, all reduced median ARRs on-treatment. Treatment failure rates were lower in patients on maintenance steroids (5%) compared with non-steroidal maintenance immunotherapy (38%) (P=0.016). 58% of patients experienced residual disability (average follow-up 61 months, visual loss in 24%). Patients with ON were less likely to have sustained disability defined by a final EDSS of ≥2 (OR 0.15, P=0.032), while those who had any myelitis were more likely to have sustained residual deficits (OR 3.56, P=0.077).
Conclusion: Relapsing MOG antibody-associated demyelination is strongly associated with ON across all age groups and ADEM in children. Patients are highly responsive to steroids, but vulnerable to relapse on steroid reduction and cessation.
Keywords: acute disseminated encephalomyelitis; myelin oligodendrocyte glycoprotein antibodies; optic neuritis; outcomes; therapy.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: Dr SR has received research funding from the National Health and Medical Research Council, the Petre Foundation and the Brain Foundation (Australia). Dr SM has received a scholarship from the National Health and Medical Research Council (Australia) and funding from the National Blood Authority IVIg grant. SB has received honoraria for attendance at advisory boards and travel sponsorship from Bayer-Schering, Biogen-Idec, Merck-Serono, Novartis and Sanofi-Genzyme, has received speakers honoraria from Biogen-Idec and Genzyme, is an investigator in clinical trials sponsored by Biogen-Idec, Novartis and Genzyme and was the recipient of an unencumbered research grant from Biogen-Idec. JL-S has accepted travel compensation from Novartis, Biogen and Merck-Serono. Her institution receives the honoraria for talks and advisory board commitment as well as research grants from Bayer Health Care, Biogen, Sanofi-Genzyme, Merck, Novartis and Teva. SR reports grants and personal fees from Sanofi-Genzyme, personal fees and departmental support from the Government of Australia, Baxter, Biogen, CSL and Merck; and departmental support from Novartis, outside the subject of the submitted work. Associate Professor FB has received research funding from the Star Scientific Foundation, The Trish Multiple Sclerosis Research Foundation, Multiple Sclerosis Research Australia and the National Health Medical Research Council (Australia). RD has received research funding from the Star Scientific Foundation, The Trish Multiple Sclerosis Research Foundation, Multiple Sclerosis Research Australia, the Petre Foundation and the National Health Medical Research Council (Australia). Dr RCD has received honoraria from Biogen-Idec as an invited speaker. Dr JLB has received compensation for education travel, honoraria for talks and advisory boards from Biogen, Teva, Merck-Serono, Sanofi-Genzyme, Novartis and Roche. Dr AC has received honoraria for attendance at advisory boards from Biogen and is an investigator in clinical trials sponsored by Biogen and Pfizer. Dr PC has accepted travel compensation from, Biogen and Merck Serono, and fellowship funding from Novartis/Biogen. Associate Professor CLF has received payment from Roche as a consultant. Dr MPM has received travel grants, speaking honoraria and unconditional research funding from Bayer, Biogen and Merck. Professor IES is supported by NHMRC Program Grant (1091593, 2016–2020) and Senior Practitioner Fellowship (1104831, 2016– 2020). IES serves on the editorial boards of Neurology and Epileptic Disorders; may accrue future revenue on a pending patent report: therapeutic compound; has received speakers honoraria from Athena Diagnostics, UCB, GSK, Eisai and Transgenomics; has received scientific advisory board honoraria from Nutricia and GSK, has received funding for travel from Athena Diagnostics, UCB and GSK and receives/has received research support from the NHMRC, ARC, NIH, Health Research Council of New Zealand, March of Dimes, the Weizmann Institute, CURE, US Department of Defense and the Perpetual Charitable Trustees. Dr EY is supported by an NHMRC Early Career Fellowship (APP1073323).
Figures
Comment in
-
Clinical course of MOG antibody-associated recurrent demyelinating diseases.J Neurol Neurosurg Psychiatry. 2018 Feb;89(2):118. doi: 10.1136/jnnp-2017-317249. Epub 2017 Nov 24. J Neurol Neurosurg Psychiatry. 2018. PMID: 29175896 No abstract available.
-
Myelin oligodendrocyte glycoprotein antibody-associated disease: characterising clinical disease.J Neurol. 2018 Aug;265(8):1950-1952. doi: 10.1007/s00415-018-8963-z. J Neurol. 2018. PMID: 29992350 Free PMC article. No abstract available.
Similar articles
-
Myelin Oligodendrocyte Glycoprotein-Associated Pediatric Central Nervous System Demyelination: Clinical Course, Neuroimaging Findings, and Response to Therapy.Neuropediatrics. 2016 Aug;47(4):245-52. doi: 10.1055/s-0036-1583184. Epub 2016 Apr 29. Neuropediatrics. 2016. PMID: 27128728
-
Clinical and MRI phenotype of children with MOG antibodies.Mult Scler. 2016 Feb;22(2):174-84. doi: 10.1177/1352458515587751. Epub 2015 Jun 3. Mult Scler. 2016. PMID: 26041801 Free PMC article.
-
MOG antibody-associated encephalomyelitis/encephalitis.Mult Scler. 2019 Oct;25(11):1427-1433. doi: 10.1177/1352458519837705. Epub 2019 Mar 25. Mult Scler. 2019. PMID: 30907249 Free PMC article. Review.
-
Disease Course and Treatment Responses in Children With Relapsing Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease.JAMA Neurol. 2018 Apr 1;75(4):478-487. doi: 10.1001/jamaneurol.2017.4601. JAMA Neurol. 2018. PMID: 29305608 Free PMC article.
-
Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease: Current Insights into the Disease Pathophysiology, Diagnosis and Management.Int J Mol Sci. 2020 Dec 24;22(1):100. doi: 10.3390/ijms22010100. Int J Mol Sci. 2020. PMID: 33374173 Free PMC article. Review.
Cited by
-
Serum Biomarkers in Neuro-Ophthalmology: When to Test.Semin Ophthalmol. 2021 May 19;36(4):322-328. doi: 10.1080/08820538.2021.1897856. Epub 2021 Mar 10. Semin Ophthalmol. 2021. PMID: 33689572 Free PMC article.
-
Rare autoimmune encephalitis presenting as fluid-attenuated inversion recovery-hyperintense lesions in anti-Myelin oligodendrocyte glycoprotein-associated encephalitis and seizures accompanied with anti-IgLON5 antibody.Quant Imaging Med Surg. 2022 Jul;12(7):4007-4012. doi: 10.21037/qims-21-1213. Quant Imaging Med Surg. 2022. PMID: 35782248 Free PMC article. No abstract available.
-
GRP78 Antibodies Are Associated With Blood-Brain Barrier Breakdown in Anti-Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disorder.Neurol Neuroimmunol Neuroinflamm. 2021 Nov 1;9(1):e1038. doi: 10.1212/NXI.0000000000001038. Print 2022 Jan. Neurol Neuroimmunol Neuroinflamm. 2021. PMID: 34725263 Free PMC article.
-
Different Characteristics of Aquaporin-4 and Myelin Oligodendrocyte Glycoprotein Antibody-Seropositive Male Optic Neuritis in China.J Ophthalmol. 2019 Apr 1;2019:4015075. doi: 10.1155/2019/4015075. eCollection 2019. J Ophthalmol. 2019. PMID: 31061727 Free PMC article.
-
Pediatric Multiple Sclerosis: Changing the Trajectory of Progression.Curr Neurol Neurosci Rep. 2023 Nov;23(11):657-669. doi: 10.1007/s11910-023-01300-3. Epub 2023 Oct 4. Curr Neurol Neurosci Rep. 2023. PMID: 37792206 Review.
References
-
- Schluesener HJ, Sobel RA, Linington C, et al. . A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J Immunol 1987;139:4016–21. - PubMed
-
- Brunner C, Lassmann H, Waehneldt TV, et al. . Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2',3'-cyclic nucleotide 3'-phosphodiesterase in the CNS of adult rats. J Neurochem 1989;52:296–304. 10.1111/j.1471-4159.1989.tb10930.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
