Cefepime-induced neurotoxicity: a systematic review
- PMID: 29137682
- PMCID: PMC5686900
- DOI: 10.1186/s13054-017-1856-1
Cefepime-induced neurotoxicity: a systematic review
Abstract
Background: Cefepime is a widely used antibiotic with neurotoxicity attributed to its ability to cross the blood-brain barrier and exhibit concentration-dependent ϒ-aminobutyric acid (GABA) antagonism. Neurotoxic symptoms include depressed consciousness, encephalopathy, aphasia, myoclonus, seizures, and coma. Data suggest that up to 15% of ICU patients treated with cefepime may experience these adverse effects. Risk factors include renal dysfunction, excessive dosing, preexisting brain injury, and elevated serum cefepime concentrations. We aimed to characterize the clinical course of cefepime neurotoxicity and response to interventions.
Methods: A librarian-assisted search identified publications describing cefepime-associated neurotoxicity from January 1980 to February 2016 using the CINAHL and MEDLINE databases. Search terms included cefepime, neurotoxicity, encephalopathy, seizures, delirium, coma, non-convulsive status epilepticus, myoclonus, confusion, aphasia, agitation, and death. Two reviewers independently assessed identified articles for eligibility and used the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) for data reporting.
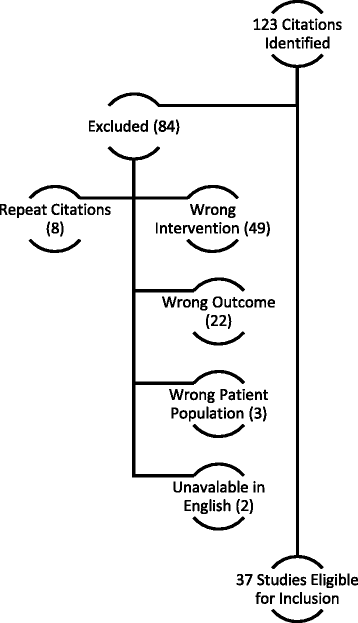
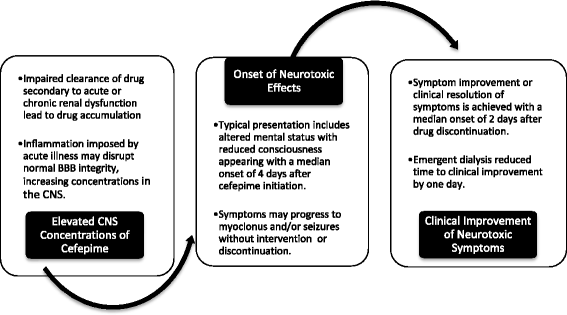
Results: Of the 123 citations identified, 37 (representing 135 patient cases) were included. Patients had a median age of 69 years, commonly had renal dysfunction (80%) and required intensive care (81% of patients with a reported location). All patients exhibited altered mental status, with reduced consciousness (47%), myoclonus (42%), and confusion (42%) being the most common symptoms. All 98 patients (73% of cohort) with electroencephalography had abnormalities, including non-convulsive status epilepticus (25%), myoclonic status epilepticus (7%), triphasic waves (40%), and focal sharp waves (39%). As per Food and Drug Administration (FDA)-approved dosing guidance, 48% of patients were overdosed; however, 26% experienced neurotoxicity despite appropriate dosing. Median cefepime serum and cerebrospinal fluid (CSF) concentrations were 45 mg/L (n = 21) and 13 mg/L (n = 4), respectively. Symptom improvement occurred in 89% of patients, and 87% survived to hospital discharge. The median delay from starting the drug to symptom onset was 4 days, and resolution occurred a median of 2 days after the intervention, which included cefepime discontinuation, antiepileptic administration, or hemodialysis.
Conclusions: Cefepime-induced neurotoxicity is challenging to recognize in the critically ill due to widely varying symptoms that are common in ICU patients. This adverse reaction can occur despite appropriate dosing, usually resolves with drug interruption, but may require additional interventions such as antiepileptic drug administration or dialysis.
Keywords: Adverse events; Blood–brain barrier; Cefepime; Cephalosporin; Coma; Intensive care units; Myoclonus; Seizures; Status epilepticus.
Conflict of interest statement
Ethics approval and consent to participate
This manuscript did not require review by the Institutional Review Board or consent, as all participants analyzed were from published data.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Cefepime neurotoxicity in the intensive care unit: a cause of severe, underappreciated encephalopathy.Crit Care. 2013 Nov 7;17(6):R264. doi: 10.1186/cc13094. Crit Care. 2013. PMID: 24200036 Free PMC article.
-
Cephalosporin-related neurotoxicity: Metabolic encephalopathy or non-convulsive status epilepticus?J Clin Neurosci. 2019 Sep;67:163-166. doi: 10.1016/j.jocn.2019.05.035. Epub 2019 Jun 11. J Clin Neurosci. 2019. PMID: 31201049
-
The neurotoxicity and safety of treatment with cefepime in patients with renal failure.Nephrol Dial Transplant. 2008 Mar;23(3):966-70. doi: 10.1093/ndt/gfm713. Epub 2008 Jan 5. Nephrol Dial Transplant. 2008. PMID: 18175786
-
Prolonged Cefepime-Induced Neurotoxicity in a Patient with End-Stage Renal Disease.Am J Case Rep. 2022 Jan 24;23:e934083. doi: 10.12659/AJCR.934083. Am J Case Rep. 2022. PMID: 35067669 Free PMC article. Review.
-
Cefepime neurotoxicity: case report, pharmacokinetic considerations, and literature review.Pharmacotherapy. 2006 Aug;26(8):1169-74. doi: 10.1592/phco.26.8.1169. Pharmacotherapy. 2006. PMID: 16863493 Review.
Cited by
-
Manual acupuncture benignly regulates blood-brain barrier disruption and reduces lipopolysaccharide loading and systemic inflammation, possibly by adjusting the gut microbiota.Front Aging Neurosci. 2022 Oct 13;14:1018371. doi: 10.3389/fnagi.2022.1018371. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36313024 Free PMC article.
-
Cefepime-Induced Encephalopathy.Cureus. 2021 Feb 4;13(2):e13125. doi: 10.7759/cureus.13125. Cureus. 2021. PMID: 33728142 Free PMC article.
-
Antimicrobial cryogel dressings towards effective wound healing.Prog Biomater. 2022 Dec;11(4):331-346. doi: 10.1007/s40204-022-00202-w. Epub 2022 Sep 19. Prog Biomater. 2022. PMID: 36123436 Free PMC article. Review.
-
Target-Controlled Infusion of Cefepime in Critically Ill Patients.Antimicrob Agents Chemother. 2019 Dec 20;64(1):e01552-19. doi: 10.1128/AAC.01552-19. Print 2019 Dec 20. Antimicrob Agents Chemother. 2019. PMID: 31685467 Free PMC article.
-
β-Lactam Pharmacokinetic/Pharmacodynamic Target Attainment in Intensive Care Unit Patients: A Prospective, Observational, Cohort Study.Antibiotics (Basel). 2023 Aug 5;12(8):1289. doi: 10.3390/antibiotics12081289. Antibiotics (Basel). 2023. PMID: 37627709 Free PMC article.
References
-
- Cefepime [package insert] Bristol-Myers Squibb Company, Princeton, NJ; 2016. Accessed Nov 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050679s036lbl.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials