Antioxidant role of autophagy in maintaining the integrity of glomerular capillaries
- PMID: 29130363
- PMCID: PMC5846506
- DOI: 10.1080/15548627.2017.1391428
Antioxidant role of autophagy in maintaining the integrity of glomerular capillaries
Abstract
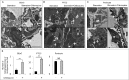
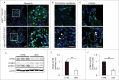
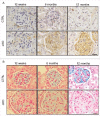
Autophagy is a lysosomal degradation system by which cytosolic materials and damaged organelles are broken down into basic components. To explore the physiological role of autophagy in glomerular endothelial cells (GEnCs), we compared the autophagic flux among cells in the kidney under starvation. Inhibition of autophagy by chloroquine administration significantly increased the number of autophagosomes or autolysosomes in GEnCs and proximal tubular cells, but not in podocytes, suggesting that the GEnCs exhibit substantial autophagic activity. Next, we analyzed endothelial and hematopoietic cell-specific atg5-deficient mice (atg5-conditional KO [cKO] mice). Glomeruli of 4-wk-old atg5-cKO mice exhibited slightly distended capillary loops accompanied by an accumulation of reactive oxygen species (ROS). Glomeruli of 8-wk-old atg5-cKO mice showed a lobular pattern with thickening of the capillary loops and mesangial matrix expansion; however, the vasculature of other organs was preserved. The atg5-cKO mice died by 12 wk of age, presumably due to pancytopenia resulting from the defect in their hematopoietic lineages. Therefore, we subjected 4-wk atg5-cKO mice to irradiation followed by bone marrow transplantation from normal littermates. Transplanted mice recapitulated the glomerular phenotypes of the atg5-cKO mice with no obvious histological changes in other organs. Twelve-mo-old transplanted mice developed mesangiolysis and glomerulosclerosis with significant deterioration of kidney function. Administration of N-acetyl-l-cysteine, a ROS scavenger, to atg5-cKO mice rescued the glomerular phenotypes. These data suggest that endothelial autophagy protects glomeruli from oxidative stress and maintains the integrity of glomerular capillaries. Enhancing endothelial autophagy may provide a novel therapeutic approach to minimizing glomerular diseases.
Keywords: Atg5; autophagic flux; autophagy; glomerular endothelial cells; oxidative stress.
Figures




Similar articles
-
Endothelial-Specific Deficiency of ATG5 (Autophagy Protein 5) Attenuates Ischemia-Related Angiogenesis.Arterioscler Thromb Vasc Biol. 2019 Jun;39(6):1137-1148. doi: 10.1161/ATVBAHA.119.309973. Arterioscler Thromb Vasc Biol. 2019. PMID: 31070476
-
Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice.J Clin Invest. 2010 Apr;120(4):1084-96. doi: 10.1172/JCI39492. J Clin Invest. 2010. PMID: 20200449 Free PMC article.
-
Protective role of podocyte autophagy against glomerular endothelial dysfunction in diabetes.Biochem Biophys Res Commun. 2020 Apr 30;525(2):319-325. doi: 10.1016/j.bbrc.2020.02.088. Epub 2020 Feb 20. Biochem Biophys Res Commun. 2020. PMID: 32089264
-
Molecular mechanisms and physiological roles of Atg5/Atg7-independent alternative autophagy.Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(6):378-385. doi: 10.2183/pjab.93.023. Proc Jpn Acad Ser B Phys Biol Sci. 2017. PMID: 28603209 Free PMC article. Review.
-
Autophagy in glomerular health and disease.Semin Nephrol. 2014 Jan;34(1):42-52. doi: 10.1016/j.semnephrol.2013.11.007. Epub 2013 Nov 22. Semin Nephrol. 2014. PMID: 24485029 Review.
Cited by
-
A Commonly Used Biocide 2-N-octyl-4-isothiazolin-3-oneInduces Blood-Brain Barrier Dysfunction via Cellular Thiol Modification and Mitochondrial Damage.Int J Mol Sci. 2021 Mar 4;22(5):2563. doi: 10.3390/ijms22052563. Int J Mol Sci. 2021. PMID: 33806369 Free PMC article.
-
Lysosomal dysfunction-induced autophagic stress in diabetic kidney disease.J Cell Mol Med. 2020 Aug;24(15):8276-8290. doi: 10.1111/jcmm.15301. Epub 2020 Jun 25. J Cell Mol Med. 2020. PMID: 32583573 Free PMC article. Review.
-
VEGF Triggers Transient Induction of Autophagy in Endothelial Cells via AMPKα1.Cells. 2020 Mar 11;9(3):687. doi: 10.3390/cells9030687. Cells. 2020. PMID: 32168879 Free PMC article.
-
TGF-β3 Induces Autophagic Activity by Increasing ROS Generation in a NOX4-Dependent Pathway.Mediators Inflamm. 2019 Dec 31;2019:3153240. doi: 10.1155/2019/3153240. eCollection 2019. Mediators Inflamm. 2019. PMID: 32082074 Free PMC article.
-
(Pro)Renin Receptor Decoy Peptide PRO20 Protects against Oxidative Renal Damage Induced by Advanced Oxidation Protein Products.Molecules. 2023 Mar 28;28(7):3017. doi: 10.3390/molecules28073017. Molecules. 2023. PMID: 37049779 Free PMC article.
References
-
- Guo F, Li X, Peng J, Tang Y, Yang Q, Liu L, Wang Z, Jiang Z, Xiao M, Ni C, et al. . Autophagy regulates vascular endothelial cell eNOS and ET-1 expression induced by laminar shear stress in an ex vivo perfused system. Ann Biomed Eng. 2014;42:1978–88. doi:10.1007/s10439-014-1033-5. PMID:24838486. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
