Early cerebellar deficits in mitochondrial biogenesis and respiratory chain complexes in the KIKO mouse model of Friedreich ataxia
- PMID: 29125827
- PMCID: PMC5719255
- DOI: 10.1242/dmm.030502
Early cerebellar deficits in mitochondrial biogenesis and respiratory chain complexes in the KIKO mouse model of Friedreich ataxia
Erratum in
-
Correction: Early cerebellar deficits in mitochondrial biogenesis and respiratory chain complexes in the KIKO mouse model of Friedreich ataxia (doi: 10.1242/dmm.030502).Dis Model Mech. 2018 Jan 29;11(1):dmm033415. doi: 10.1242/dmm.033415. Dis Model Mech. 2018. PMID: 29419392 Free PMC article. No abstract available.
Abstract
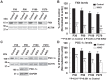
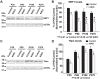
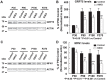
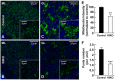
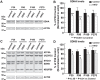
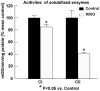
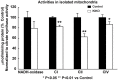
Friedreich ataxia (FRDA), the most common recessive inherited ataxia, results from deficiency of frataxin, a small mitochondrial protein crucial for iron-sulphur cluster formation and ATP production. Frataxin deficiency is associated with mitochondrial dysfunction in FRDA patients and animal models; however, early mitochondrial pathology in FRDA cerebellum remains elusive. Using frataxin knock-in/knockout (KIKO) mice and KIKO mice carrying the mitoDendra transgene, we show early cerebellar deficits in mitochondrial biogenesis and respiratory chain complexes in this FRDA model. At asymptomatic stages, the levels of PGC-1α (PPARGC1A), the mitochondrial biogenesis master regulator, are significantly decreased in cerebellar homogenates of KIKO mice compared with age-matched controls. Similarly, the levels of the PGC-1α downstream effectors, NRF1 and Tfam, are significantly decreased, suggesting early impaired cerebellar mitochondrial biogenesis pathways. Early mitochondrial deficiency is further supported by significant reduction of the mitochondrial markers GRP75 (HSPA9) and mitofusin-1 in the cerebellar cortex. Moreover, the numbers of Dendra-labeled mitochondria are significantly decreased in cerebellar cortex, confirming asymptomatic cerebellar mitochondrial biogenesis deficits. Functionally, complex I and II enzyme activities are significantly reduced in isolated mitochondria and tissue homogenates from asymptomatic KIKO cerebella. Structurally, levels of the complex I core subunit NUDFB8 and complex II subunits SDHA and SDHB are significantly lower than those in age-matched controls. These results demonstrate complex I and II deficiency in KIKO cerebellum, consistent with defects identified in FRDA patient tissues. Thus, our findings identify early cerebellar mitochondrial biogenesis deficits as a potential mediator of cerebellar dysfunction and ataxia, thereby providing a potential therapeutic target for early intervention of FRDA.
Keywords: Cerebellum; Friedreich ataxia; Mitochondrial biogenesis; Neurodegenerative diseases; Respiratory chain complex.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

Similar articles
-
Early VGLUT1-specific parallel fiber synaptic deficits and dysregulated cerebellar circuit in the KIKO mouse model of Friedreich ataxia.Dis Model Mech. 2017 Dec 19;10(12):1529-1538. doi: 10.1242/dmm.030049. Dis Model Mech. 2017. PMID: 29259026 Free PMC article.
-
PPAR gamma agonist leriglitazone improves frataxin-loss impairments in cellular and animal models of Friedreich Ataxia.Neurobiol Dis. 2021 Jan;148:105162. doi: 10.1016/j.nbd.2020.105162. Epub 2020 Nov 7. Neurobiol Dis. 2021. PMID: 33171227
-
GRP75 overexpression rescues frataxin deficiency and mitochondrial phenotypes in Friedreich ataxia cellular models.Hum Mol Genet. 2019 May 15;28(10):1594-1607. doi: 10.1093/hmg/ddy448. Hum Mol Genet. 2019. PMID: 30590615 Free PMC article.
-
Skeletal Muscle Involvement in Friedreich Ataxia.Int J Mol Sci. 2024 Sep 13;25(18):9915. doi: 10.3390/ijms25189915. Int J Mol Sci. 2024. PMID: 39337401 Free PMC article. Review.
-
Friedreich ataxia: an update on animal models, frataxin function and therapies.Adv Exp Med Biol. 2009;652:247-61. doi: 10.1007/978-90-481-2813-6_17. Adv Exp Med Biol. 2009. PMID: 20225031 Review.
Cited by
-
Cerebellar Pathology in an Inducible Mouse Model of Friedreich Ataxia.Front Neurosci. 2022 Mar 24;16:819569. doi: 10.3389/fnins.2022.819569. eCollection 2022. Front Neurosci. 2022. PMID: 35401081 Free PMC article.
-
An open-label pilot study of recombinant granulocyte-colony stimulating factor in Friedreich's ataxia.Nat Commun. 2022 Aug 9;13(1):4655. doi: 10.1038/s41467-022-31450-w. Nat Commun. 2022. PMID: 35945193 Free PMC article. Clinical Trial.
-
Transcriptional profiling of isogenic Friedreich ataxia neurons and effect of an HDAC inhibitor on disease signatures.J Biol Chem. 2019 Feb 8;294(6):1846-1859. doi: 10.1074/jbc.RA118.006515. Epub 2018 Dec 14. J Biol Chem. 2019. PMID: 30552117 Free PMC article.
-
Butyrate prevents visceral adipose tissue inflammation and metabolic alterations in a Friedreich's ataxia mouse model.iScience. 2023 Aug 28;26(10):107713. doi: 10.1016/j.isci.2023.107713. eCollection 2023 Oct 20. iScience. 2023. PMID: 37701569 Free PMC article.
-
Nuclear Factor Erythroid-2-Related Factor 2 Signaling in the Neuropathophysiology of Inherited Metabolic Disorders.Front Cell Neurosci. 2021 Nov 26;15:785057. doi: 10.3389/fncel.2021.785057. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34955754 Free PMC article. Review.
References
-
- Aleshin S., Strokin M., Sergeeva M. and Reiser G. (2013). Peroxisome proliferator-activated receptor (PPAR)beta/delta, a possible nexus of PPARalpha- and PPARgamma-dependent molecular pathways in neurodegenerative diseases: review and novel hypotheses. Neurochem. Int. 63, 322-330. 10.1016/j.neuint.2013.06.012 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

