Kinetic Control of Quorum Sensing in Pseudomonas aeruginosa by Multidrug Efflux Pumps
- PMID: 29115136
- PMCID: PMC5807214
- DOI: 10.1021/acsinfecdis.7b00160
Kinetic Control of Quorum Sensing in Pseudomonas aeruginosa by Multidrug Efflux Pumps
Abstract
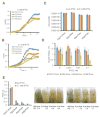
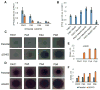
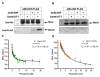
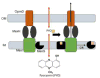
Pseudomonas aeruginosa is an important human pathogen, the physiology and virulence of which are under the control of quorum sensing signals. These signals often have dual roles, functioning as toxins to some cells and as oxidative-stress protectors for their producer cells. Hence, their internal and external concentrations should be tightly controlled. In this study, we analyzed the interplay between the multidrug efflux transporters MexEF-OprN and MexG/HI-OpmD in quorum sensing of P. aeruginosa. We found that the two transporters have overlapping substrate specificities but different efficiencies. When overproduced, both MexEF-OprN and MexG/HI-OpmD provide clinical levels of resistance to diverse fluoroquinolones and protect P. aeruginosa against toxic phenazines. However, this similarity is enabled by synergistic interactions with the outer membrane. In hyperporinated cells, MexG/HI-OpmD is saturated by much lower concentrations of fluoroquinolones but is more efficient than MexEF-OprN in efflux of phenazines. Unlike MexEF-OprN, mutational inactivation of MexG/HI-OpmD reduces the levels of pyocyanin and makes P. aeruginosa cells hypersusceptible to phenazines. Our results further show that MexG binds pyocyanin, physically associates with MexHI, and represses the activity of the transporter, revealing a negative regulatory role of this protein. We conclude that differences in kinetic properties of transporters are critical to maintain proper intra- and extracellular concentrations of phenazines and other signaling molecules and that MexG/HI-OpmD controls the steady state in the synthesis and secretion of phenazines.
Keywords: antibiotic resistance; efflux constant; hyperporination; outer membrane barrier; permeation; phenazines.
Figures


Similar articles
-
Toxic Electrophiles Induce Expression of the Multidrug Efflux Pump MexEF-OprN in Pseudomonas aeruginosa through a Novel Transcriptional Regulator, CmrA.Antimicrob Agents Chemother. 2017 Jul 25;61(8):e00585-17. doi: 10.1128/AAC.00585-17. Print 2017 Aug. Antimicrob Agents Chemother. 2017. PMID: 28507116 Free PMC article.
-
Uncoupled Quorum Sensing Modulates the Interplay of Virulence and Resistance in a Multidrug-Resistant Clinical Pseudomonas aeruginosa Isolate Belonging to the MLST550 Clonal Complex.Antimicrob Agents Chemother. 2019 Mar 27;63(4):e01944-18. doi: 10.1128/AAC.01944-18. Print 2019 Apr. Antimicrob Agents Chemother. 2019. PMID: 30670423 Free PMC article.
-
Overexpression of the multidrug efflux pumps MexCD-OprJ and MexEF-OprN is associated with a reduction of type III secretion in Pseudomonas aeruginosa.J Bacteriol. 2005 Feb;187(4):1384-91. doi: 10.1128/JB.187.4.1384-1391.2005. J Bacteriol. 2005. PMID: 15687203 Free PMC article.
-
Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms.J Mol Microbiol Biotechnol. 2001 Apr;3(2):255-64. J Mol Microbiol Biotechnol. 2001. PMID: 11321581 Review.
-
Efflux as a mechanism of resistance to antimicrobials in Pseudomonas aeruginosa and related bacteria: unanswered questions.Genet Mol Res. 2003 Mar 31;2(1):48-62. Genet Mol Res. 2003. PMID: 12917802 Review.
Cited by
-
The Natural Product Elegaphenone Potentiates Antibiotic Effects against Pseudomonas aeruginosa.Angew Chem Int Ed Engl. 2019 Jun 17;58(25):8581-8584. doi: 10.1002/anie.201903472. Epub 2019 May 16. Angew Chem Int Ed Engl. 2019. PMID: 30969469 Free PMC article.
-
Alternating Dynamics of oriC, SMC, and MksBEF in Segregation of Pseudomonas aeruginosa Chromosome.mSphere. 2020 Sep 9;5(5):e00238-20. doi: 10.1128/mSphere.00238-20. mSphere. 2020. PMID: 32907947 Free PMC article.
-
Molecular Properties That Define the Activities of Antibiotics in Escherichia coli and Pseudomonas aeruginosa.ACS Infect Dis. 2018 Aug 10;4(8):1223-1234. doi: 10.1021/acsinfecdis.8b00036. Epub 2018 May 25. ACS Infect Dis. 2018. PMID: 29756762 Free PMC article.
-
From the soil to the clinic: the impact of microbial secondary metabolites on antibiotic tolerance and resistance.Nat Rev Microbiol. 2022 Mar;20(3):129-142. doi: 10.1038/s41579-021-00620-w. Epub 2021 Sep 16. Nat Rev Microbiol. 2022. PMID: 34531577 Free PMC article. Review.
-
MP4: a machine learning based classification tool for prediction and functional annotation of pathogenic proteins from metagenomic and genomic datasets.BMC Bioinformatics. 2022 Nov 28;23(1):507. doi: 10.1186/s12859-022-05061-7. BMC Bioinformatics. 2022. PMID: 36443666 Free PMC article.
References
-
- World Health, O. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. 2017.
-
- Zgurskaya HI, Nikaido H. Multidrug resistance mechanisms: drug efflux across two membranes. Mol Microbiol. 2000;37(2):219–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

