Vinpocetine alleviate cerebral ischemia/reperfusion injury by down-regulating TLR4/MyD88/NF-κB signaling
- PMID: 29113305
- PMCID: PMC5655200
- DOI: 10.18632/oncotarget.20699
Vinpocetine alleviate cerebral ischemia/reperfusion injury by down-regulating TLR4/MyD88/NF-κB signaling
Abstract
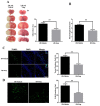
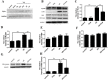
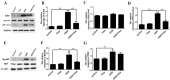
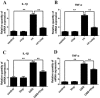
Inflammatory responses play crucial roles in cerebral ischemia/reperfusion injury. Toll-like receptor 4 (TLR4) is an important mediator of the neuroinflammatory response to cerebral ischemia/reperfusion injury. Vinpocetine is a derivative of the alkaloid vincamine and exerts an anti-inflammatory effect by inhibiting NF-κB activation. However, the effects of vinpocetine on pathways upstream of NF-κB signaling, such as TLR4, have not been fully elucidated. Here, we used mouse middle cerebral artery occlusion (MCAO) and cell-based oxygen-glucose deprivation (OGD) models to evaluate the therapeutic effects and mechanisms of vinpocetine treatment. The vinpocetine treatment significantly reduced mice cerebral infarct volumes and neurological scores. Moreover, the numbers of TUNEL+ and Fluoro-Jade B+ cells were significantly decreased in the ischemic brain tissues after vinpocetine treatment. In the OGD model, the vinpocetine treatment also increased the viability of cultured cortical neurons. Interestingly, vinpocetine exerted a neuroprotective effect on the mouse MCAO model and cell-based OGD model by inhibiting TLR4-mediated inflammatory responses and decreasing proinflammatory cytokine release through the MyD88-dependent signaling pathway, independent of TRIF signaling pathway. In conclusion, vinpocetine exerts anti-inflammatory effects to ameliorate cerebral ischemia/reperfusion injury in vitro and in vivo. Vinpocetine may inhibit inflammatory responses through the TLR4/MyD88/NF-κB signaling pathway, independent of TRIF-mediated inflammatory responses. Thus, vinpocetine may be an attractive therapeutic candidate for the treatment of ischemic cerebral injury or other inflammatory diseases.
Keywords: cerebral ischemia/reperfusion; ischemic stroke; middle cerebral artery occlusion; toll-like receptor 4; vinpocetine.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have no competing interests to declare.
Figures

References
-
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. - PMC - PubMed
-
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, American Heart Association Statistics Committee and Stroke Statistics Subcommittee et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. - PMC - PubMed
-
- Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. - PubMed
-
- Bonaventura A, Montecucco F, Dallegri F. Update on the effects of treatment with recombinant tissue-type plasminogen activator (rt-PA) in acute ischemic stroke. Expert Opin Biol Ther. 2016;16:1323–40. - PubMed
-
- Murray KN, Buggey HF, Denes A, Allan SM. Systemic immune activation shapes stroke outcome. Mol Cell Neurosci. 2013;53:14–25. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

