ER-mitochondria tethering by PDZD8 regulates Ca2+ dynamics in mammalian neurons
- PMID: 29097544
- PMCID: PMC5818999
- DOI: 10.1126/science.aan6009
ER-mitochondria tethering by PDZD8 regulates Ca2+ dynamics in mammalian neurons
Abstract
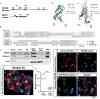
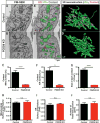
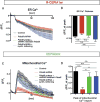
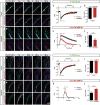
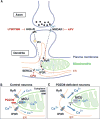
Interfaces between organelles are emerging as critical platforms for many biological responses in eukaryotic cells. In yeast, the ERMES complex is an endoplasmic reticulum (ER)-mitochondria tether composed of four proteins, three of which contain a SMP (synaptotagmin-like mitochondrial-lipid binding protein) domain. No functional ortholog for any ERMES protein has been identified in metazoans. Here, we identified PDZD8 as an ER protein present at ER-mitochondria contacts. The SMP domain of PDZD8 is functionally orthologous to the SMP domain found in yeast Mmm1. PDZD8 was necessary for the formation of ER-mitochondria contacts in mammalian cells. In neurons, PDZD8 was required for calcium ion (Ca2+) uptake by mitochondria after synaptically induced Ca2+-release from ER and thereby regulated cytoplasmic Ca2+ dynamics. Thus, PDZD8 represents a critical ER-mitochondria tethering protein in metazoans. We suggest that ER-mitochondria coupling is involved in the regulation of dendritic Ca2+ dynamics in mammalian neurons.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
Comment in
-
Mediating ER-mitochondrial cross-talk.Science. 2017 Nov 3;358(6363):591-592. doi: 10.1126/science.aaq0141. Science. 2017. PMID: 29097535 Free PMC article. No abstract available.
-
The Secret of the Kissing Cousins: an ER-mitochondrial tethering protein regulates Ca2+ crosstalk in mammalian neurons.Cardiovasc Res. 2018 Mar 1;114(3):e17-e18. doi: 10.1093/cvr/cvy020. Cardiovasc Res. 2018. PMID: 29481649 No abstract available.
Similar articles
-
Protrudin and PDZD8 contribute to neuronal integrity by promoting lipid extraction required for endosome maturation.Nat Commun. 2020 Sep 11;11(1):4576. doi: 10.1038/s41467-020-18413-9. Nat Commun. 2020. PMID: 32917905 Free PMC article.
-
PDZD8 is not the 'functional ortholog' of Mmm1, it is a paralog.F1000Res. 2018 Jul 16;7:1088. doi: 10.12688/f1000research.15523.1. eCollection 2018. F1000Res. 2018. PMID: 30109028 Free PMC article.
-
PDZD8 interacts with Protrudin and Rab7 at ER-late endosome membrane contact sites associated with mitochondria.Nat Commun. 2020 Jul 20;11(1):3645. doi: 10.1038/s41467-020-17451-7. Nat Commun. 2020. PMID: 32686675 Free PMC article.
-
New functions of mitochondria associated membranes in cellular signaling.Biochim Biophys Acta. 2014 Oct;1843(10):2253-62. doi: 10.1016/j.bbamcr.2014.03.009. Epub 2014 Mar 15. Biochim Biophys Acta. 2014. PMID: 24642268 Review.
-
The ERMES complex and ER-mitochondria connections.Biochem Soc Trans. 2012 Apr;40(2):445-50. doi: 10.1042/BST20110758. Biochem Soc Trans. 2012. PMID: 22435828 Review.
Cited by
-
Endoplasmic reticulum & mitochondrial calcium homeostasis: The interplay with viruses.Mitochondrion. 2021 May;58:227-242. doi: 10.1016/j.mito.2021.03.008. Epub 2021 Mar 26. Mitochondrion. 2021. PMID: 33775873 Free PMC article.
-
MTCH2 cooperates with MFN2 and lysophosphatidic acid synthesis to sustain mitochondrial fusion.EMBO Rep. 2024 Jan;25(1):45-67. doi: 10.1038/s44319-023-00009-1. Epub 2023 Dec 14. EMBO Rep. 2024. PMID: 38177900 Free PMC article.
-
ER-Mitochondria Communication in Cells of the Innate Immune System.Cells. 2019 Sep 15;8(9):1088. doi: 10.3390/cells8091088. Cells. 2019. PMID: 31540165 Free PMC article. Review.
-
Targeting ER-Mitochondria Signaling as a Therapeutic Target for Frontotemporal Dementia and Related Amyotrophic Lateral Sclerosis.Front Cell Dev Biol. 2022 May 27;10:915931. doi: 10.3389/fcell.2022.915931. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35693938 Free PMC article. Review.
-
Trazodone rescues dysregulated synaptic and mitochondrial nascent proteomes in prion neurodegeneration.Brain. 2024 Feb 1;147(2):649-664. doi: 10.1093/brain/awad313. Brain. 2024. PMID: 37703312 Free PMC article.
References
-
- Rizzuto R, et al. Science. 1998;280:1763–1766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

