Update on Vaccine-Derived Polioviruses - Worldwide, January 2016-June 2017
- PMID: 29095803
- PMCID: PMC5689216
- DOI: 10.15585/mmwr.mm6643a6
Update on Vaccine-Derived Polioviruses - Worldwide, January 2016-June 2017
Abstract
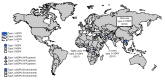
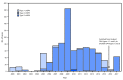
In 1988, the World Health Assembly launched the Global Polio Eradication Initiative (GPEI) (1). Among the three wild poliovirus (WPV) serotypes, only type 1 (WPV1) has been detected since 2012. Since 2014, detection of WPV1 has been limited to three countries, with 37 cases in 2016 and 11 cases in 2017 as of September 27. The >99.99% decline worldwide in polio cases since the launch of the GPEI is attributable to the extensive use of the live, attenuated oral poliovirus vaccine (OPV) in mass vaccination campaigns and comprehensive national routine immunization programs. Despite its well-established safety record, OPV use can be associated with rare emergence of genetically divergent vaccine-derived polioviruses (VDPVs) whose genetic drift from the parental OPV strains indicates prolonged replication or circulation (2). VDPVs can also emerge among persons with primary immunodeficiencies (PIDs). Immunodeficiency-associated VDPVs (iVDPVs) can replicate for years in some persons with PIDs. In addition, circulating vaccine-derived polioviruses (cVDPVs) can emerge very rarely among immunologically normal vaccine recipients and their contacts in areas with inadequate OPV coverage and can cause outbreaks of paralytic polio. This report updates previous summaries regarding VDPVs (3). During January 2016-June 2017, new cVDPV outbreaks were identified, including two in the Democratic Republic of the Congo (DRC) (eight cases), and another in Syria (35 cases), whereas the circulation of cVDPV type 2 (cVDPV2) in Nigeria resulted in cVDPV2 detection linked to a previous emergence. The last confirmed case from the 2015-2016 cVDPV type 1 (cVDPV1) outbreak in Laos occurred in January 2016. Fourteen newly identified persons in 10 countries were found to excrete iVDPVs, and three previously reported patients in the United Kingdom and Iran (3) were still excreting type 2 iVDPV (iVDPV2) during the reporting period. Ambiguous VDPVs (aVDPVs), isolates that cannot be classified definitively, were found among immunocompetent persons and environmental samples in 10 countries. Cessation of all OPV use after certification of polio eradication will eliminate the risk for new VDPV infections.
Conflict of interest statement
Figures
Similar articles
-
Update on Vaccine-Derived Polioviruses - Worldwide, January 2014-March 2015.MMWR Morb Mortal Wkly Rep. 2015 Jun 19;64(23):640-6. MMWR Morb Mortal Wkly Rep. 2015. PMID: 26086635 Free PMC article.
-
Update on Vaccine-Derived Polioviruses - Worldwide, January 2017-June 2018.MMWR Morb Mortal Wkly Rep. 2018 Oct 26;67(42):1189-1194. doi: 10.15585/mmwr.mm6742a5. MMWR Morb Mortal Wkly Rep. 2018. PMID: 30359342 Free PMC article.
-
Update on Vaccine-Derived Polioviruses - Worldwide, January 2015-May 2016.MMWR Morb Mortal Wkly Rep. 2016 Aug 5;65(30):763-9. doi: 10.15585/mmwr.mm6530a3. MMWR Morb Mortal Wkly Rep. 2016. PMID: 27491079
-
Vaccine-derived polioviruses.J Infect Dis. 2014 Nov 1;210 Suppl 1:S283-93. doi: 10.1093/infdis/jiu295. J Infect Dis. 2014. PMID: 25316847 Review.
-
Circulating vaccine-derived polioviruses: current state of knowledge.Bull World Health Organ. 2004 Jan;82(1):16-23. Epub 2004 Feb 26. Bull World Health Organ. 2004. PMID: 15106296 Free PMC article. Review.
Cited by
-
Monitoring the evolution of vaccine-derived poliovirus in East and Southern African countries, 2010 - 2021.Pan Afr Med J. 2024 Jan 25;47:31. doi: 10.11604/pamj.2024.47.31.39945. eCollection 2024. Pan Afr Med J. 2024. PMID: 38586072 Free PMC article.
-
Strategic Response to an Outbreak of Circulating Vaccine-Derived Poliovirus Type 2 - Syria, 2017-2018.MMWR Morb Mortal Wkly Rep. 2018 Jun 22;67(24):690-694. doi: 10.15585/mmwr.mm6724a5. MMWR Morb Mortal Wkly Rep. 2018. PMID: 29927908 Free PMC article.
-
Update on Vaccine-Derived Poliovirus Outbreaks - Democratic Republic of the Congo and Horn of Africa, 2017-2018.MMWR Morb Mortal Wkly Rep. 2019 Mar 8;68(9):225-230. doi: 10.15585/mmwr.mm6809a2. MMWR Morb Mortal Wkly Rep. 2019. PMID: 30845121 Free PMC article.
-
Poliovirus containment risks and their management.Future Virol. 2018 Aug 10;13(9):617-628. doi: 10.2217/fvl-2018-0079. Future Virol. 2018. PMID: 33598044 Free PMC article.
-
Polio endgame risks and the possibility of restarting the use of oral poliovirus vaccine.Expert Rev Vaccines. 2018 Aug;17(8):739-751. doi: 10.1080/14760584.2018.1506333. Epub 2018 Aug 9. Expert Rev Vaccines. 2018. PMID: 30056767 Free PMC article. Review.
References
-
- Hampton LM, Farrell M, Ramirez-Gonzalez A, et al.; Immunization Systems Management Group of the Global Polio Eradication Initiative. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine—worldwide, 2016. MMWR Morb Mortal Wkly Rep 2016;65:934–8. 10.15585/mmwr.mm6535a3 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

