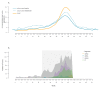
Low interim influenza vaccine effectiveness, Australia, 1 May to 24 September 2017
- PMID: 29090681
- PMCID: PMC5718387
- DOI: 10.2807/1560-7917.ES.2017.22.43.17-00707
Low interim influenza vaccine effectiveness, Australia, 1 May to 24 September 2017
Abstract
In 2017, influenza seasonal activity was high in the southern hemisphere. We present interim influenza vaccine effectiveness (VE) estimates from Australia. Adjusted VE was low overall at 33% (95% confidence interval (CI): 17 to 46), 50% (95% CI: 8 to 74) for A(H1)pdm09, 10% (95% CI: -16 to 31) for A(H3) and 57% (95% CI: 41 to 69) for influenza B. For A(H3), VE was poorer for those vaccinated in the current and prior seasons.
Keywords: Australia; epidemiology; influenza; influenza-like illness - ILI; surveillance; vaccines and immunisation; viral infections.
Conflict of interest statement
Figures
Similar articles
-
Vaccine effectiveness in preventing laboratory-confirmed influenza in primary care patients in a season of co-circulation of influenza A(H1N1)pdm09, B and drifted A(H3N2), I-MOVE Multicentre Case-Control Study, Europe 2014/15.Euro Surveill. 2016;21(7):pii=30139. doi: 10.2807/1560-7917.ES.2016.21.7.30139. Euro Surveill. 2016. PMID: 26924024
-
Heterogeneity in influenza seasonality and vaccine effectiveness in Australia, Chile, New Zealand and South Africa: early estimates of the 2019 influenza season.Euro Surveill. 2019 Nov;24(45):1900645. doi: 10.2807/1560-7917.ES.2019.24.45.1900645. Euro Surveill. 2019. PMID: 31718744 Free PMC article.
-
Component-specific effectiveness of trivalent influenza vaccine as monitored through a sentinel surveillance network in Canada, 2006-2007.J Infect Dis. 2009 Jan 15;199(2):168-79. doi: 10.1086/595862. J Infect Dis. 2009. PMID: 19086914
-
A comprehensive review of the epidemiology and disease burden of Influenza B in 9 European countries.Hum Vaccin Immunother. 2016 Apr 2;12(4):993-1002. doi: 10.1080/21645515.2015.1111494. Epub 2016 Feb 18. Hum Vaccin Immunother. 2016. PMID: 26890005 Free PMC article. Review.
-
Spotlight influenza: Laboratory-confirmed seasonal influenza in people with acute respiratory illness: a literature review and meta-analysis, WHO European Region, 2004 to 2017.Euro Surveill. 2021 Sep;26(39):2000343. doi: 10.2807/1560-7917.ES.2021.26.39.2000343. Euro Surveill. 2021. PMID: 34596019 Free PMC article. Review.
Cited by
-
Opposing Effects of Prior Infection versus Prior Vaccination on Vaccine Immunogenicity against Influenza A(H3N2) Viruses.Viruses. 2022 Feb 25;14(3):470. doi: 10.3390/v14030470. Viruses. 2022. PMID: 35336877 Free PMC article.
-
Genetic and antigenic characterisation of influenza A(H3N2) viruses isolated in Yokohama during the 2016/17 and 2017/18 influenza seasons.Euro Surveill. 2019 Feb;24(6):1800467. doi: 10.2807/1560-7917.ES.2019.24.6.1800467. Euro Surveill. 2019. PMID: 30755292 Free PMC article.
-
Diagnostic accuracy of an app-guided, self-administered test for influenza among individuals presenting to general practice with influenza-like illness: study protocol.BMJ Open. 2020 Nov 19;10(11):e036298. doi: 10.1136/bmjopen-2019-036298. BMJ Open. 2020. PMID: 33444172 Free PMC article. Clinical Trial.
-
On field vaccine effectiveness in three periods of 2018/2019 influenza season in Emilia-Romagna Region.Acta Biomed. 2019 Sep 13;90(9-S):21-27. doi: 10.23750/abm.v90i9-S.8699. Acta Biomed. 2019. PMID: 31517886 Free PMC article.
-
A single-shot adenoviral vaccine provides hemagglutinin stalk-mediated protection against heterosubtypic influenza challenge in mice.Mol Ther. 2022 May 4;30(5):2024-2047. doi: 10.1016/j.ymthe.2022.01.011. Epub 2022 Jan 7. Mol Ther. 2022. PMID: 34999208 Free PMC article.
References
-
- Australian Government. Department of Health. Australian Influenza Surveillance Report No 10 – week ending 29 September 2017. Canberra: Department of Health; 2017. Available from: http://www.health.gov.au/internet/main/publishing.nsf/Content/ozflu-surv...
-
- NSW Government. Health. Influenza Surveillance Report, Week 39: 25 September to 1 October 2017. North Sydney: NSW Government. [Accessed 25 Oct 2017]. Available from: http://www.health.nsw.gov.au/Infectious/Influenza/Publications/2017/week...
-
- World Health Organization (WHO). Recommended composition of influenza virus vaccines for use in the 2017 southern hemisphere influenza season. Geneva: WHO; 29 Sep 2016. Available from: http://www.who.int/influenza/vaccines/virus/recommendations/2017_south/en/
-
- Australian Government. Department of Health. Therapeutic Goods Administration. 2017 seasonal influenza vaccines. Symonston: Therapeutic Goods Administration; 6 Mar 2017. Available from: https://www.tga.gov.au/media-release/2017-seasonal-influenza-vaccines
-
- World Health Organization (WHO). Recommended composition of influenza virus vaccines for use in the 2017-2018 northern hemisphere influenza season. Geneva: WHO; 2017. Available from: http://www.who.int/influenza/vaccines/virus/recommendations/2017_18_nort...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical