Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity
- PMID: 29089479
- PMCID: PMC5663717
- DOI: 10.1038/s41467-017-01150-x
Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity
Abstract
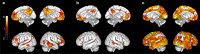
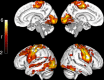
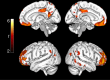
It is not known exactly where amyloid-β (Aβ) fibrils begin to accumulate in individuals with Alzheimer's disease (AD). Recently, we showed that abnormal levels of Aβ42 in cerebrospinal fluid (CSF) can be detected before abnormal amyloid can be detected using PET in individuals with preclinical AD. Using these approaches, here we identify the earliest preclinical AD stage in subjects from the ADNI and BioFINDER cohorts. We show that Aβ accumulation preferentially starts in the precuneus, medial orbitofrontal, and posterior cingulate cortices, i.e., several of the core regions of the default mode network (DMN). This early pattern of Aβ accumulation is already evident in individuals with normal Aβ42 in the CSF and normal amyloid PET who subsequently convert to having abnormal CSF Aβ42. The earliest Aβ accumulation is further associated with hypoconnectivity within the DMN and between the DMN and the frontoparietal network, but not with brain atrophy or glucose hypometabolism. Our results suggest that Aβ fibrils start to accumulate predominantly within certain parts of the DMN in preclinical AD and already then affect brain connectivity.
Conflict of interest statement
S.P., M.S., O.S., N.M., and E.S. report no competing financial interests. K.B. has served as a consultant or at advisory boards for Alzheon, Eli Lilly, Fujirebio Europe, I.B.L. International, Novartis, and Roche Diagnostics. K.B. and H.Z. are co-founders of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg. S.L. has consulted for Biogen, Synarc, and Genentech. W.J. serves as a consultant to Bioclinica, Genentech, and Novartis pharmaceuticals. O.H. has acquired research support (for the institution) from Roche, GE Healthcare, Biogen, AVID Radiopharmaceuticals, Fujirebio, and Euroimmun and in the past 2 years, he has received consultancy or speaker fees (paid to the institution) from Lilly, Roche, and Fujirebio. 18F-flutemetamol was generously provided by GE Healthcare in the BioFINDER study.
Figures

Similar articles
-
Cerebrospinal fluid lactate levels and brain [18F]FDG PET hypometabolism within the default mode network in Alzheimer's disease.Eur J Nucl Med Mol Imaging. 2016 Oct;43(11):2040-9. doi: 10.1007/s00259-016-3417-2. Epub 2016 May 25. Eur J Nucl Med Mol Imaging. 2016. PMID: 27221635
-
Phases of Hyperconnectivity and Hypoconnectivity in the Default Mode and Salience Networks Track with Amyloid and Tau in Clinically Normal Individuals.J Neurosci. 2017 Apr 19;37(16):4323-4331. doi: 10.1523/JNEUROSCI.3263-16.2017. Epub 2017 Mar 17. J Neurosci. 2017. PMID: 28314821 Free PMC article.
-
Cognition, brain atrophy, and cerebrospinal fluid biomarkers changes from preclinical to dementia stage of Alzheimer's disease and the influence of apolipoprotein e.J Alzheimers Dis. 2015;45(1):253-68. doi: 10.3233/JAD-142451. J Alzheimers Dis. 2015. PMID: 25524955
-
A review of the default mode network in aging and dementia based on molecular imaging.Rev Neurosci. 2012 Feb 28;23(3):263-8. doi: 10.1515/revneuro-2012-0029. Rev Neurosci. 2012. PMID: 22752783 Review.
-
[Default mode network and Alzheimer's disease].Rev Med Chil. 2013 Mar;141(3):375-80. doi: 10.4067/S0034-98872013000300014. Rev Med Chil. 2013. PMID: 23900330 Review. Spanish.
Cited by
-
A Scoping Review of Neuromodulation Techniques in Neurodegenerative Diseases: A Useful Tool for Clinical Practice?Medicina (Kaunas). 2021 Feb 27;57(3):215. doi: 10.3390/medicina57030215. Medicina (Kaunas). 2021. PMID: 33673455 Free PMC article.
-
Early memory deficits and extensive brain network disorganization in the AppNL-F/MAPT double knock-in mouse model of familial Alzheimer's disease.Aging Brain. 2022 Jun 2;2:100042. doi: 10.1016/j.nbas.2022.100042. eCollection 2022. Aging Brain. 2022. PMID: 36908877 Free PMC article.
-
Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases.Brain. 2019 Sep 1;142(9):2558-2571. doi: 10.1093/brain/awz193. Brain. 2019. PMID: 31327002 Free PMC article. Review.
-
Increased posterior default mode network activity and structural connectivity in young adult APOE-ε4 carriers: a multimodal imaging investigation.Neurobiol Aging. 2019 Jan;73:82-91. doi: 10.1016/j.neurobiolaging.2018.08.026. Epub 2018 Sep 22. Neurobiol Aging. 2019. PMID: 30339963 Free PMC article.
-
Tauopathy in veterans with long-term posttraumatic stress disorder and traumatic brain injury.Eur J Nucl Med Mol Imaging. 2019 May;46(5):1139-1151. doi: 10.1007/s00259-018-4241-7. Epub 2019 Jan 7. Eur J Nucl Med Mol Imaging. 2019. PMID: 30617964 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

