Bioactivity Potential of Prunus spinosa L. Flower Extracts: Phytochemical Profiling, Cellular Safety, Pro-inflammatory Enzymes Inhibition and Protective Effects Against Oxidative Stress In Vitro
- PMID: 29085295
- PMCID: PMC5649189
- DOI: 10.3389/fphar.2017.00680
Bioactivity Potential of Prunus spinosa L. Flower Extracts: Phytochemical Profiling, Cellular Safety, Pro-inflammatory Enzymes Inhibition and Protective Effects Against Oxidative Stress In Vitro
Abstract
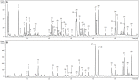
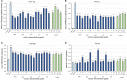
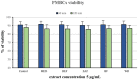
Flower extracts of Prunus spinosa L. (blackthorn)-a traditional medicinal plant of Central and Eastern Europe indicated for the treatment of urinary tract disorders, inflammation, and adjunctive therapy of cardiovascular diseases-were evaluated in terms of chemical composition, antioxidant activity, potential anti-inflammatory effects, and cellular safety in function of fractionated extraction. The UHPLC-PDA-ESI-MS3 fingerprinting led to full or partial identification of 57 marker constituents (36 new for the flowers), mostly flavonoids, A-type proanthocyanidins, and phenolic acids, and provided the basis for authentication and standardization of the flower extracts. With the contents up to 584.07 mg/g dry weight (dw), 490.63, 109.43, and 66.77 mg/g dw of total phenolics (TPC), flavonoids, proanthocyanidins, and phenolic acids, respectively, the extracts were proven to be rich sources of polyphenols. In chemical in vitro tests of antioxidant (DPPH, FRAP, TBARS) and enzyme (lipoxygenase and hyaluronidase) inhibitory activity, the extracts effects were profound, dose-, phenolic-, and extraction solvent-dependent. Moreover, at in vivo-relevant levels (1-5 μg/mL) the extracts effectively protected the human plasma components against peroxynitrite-induced damage (reduced the levels of oxidative stress biomarkers: 3-nitrotyrosine, lipid hydroperoxides, and thiobarbituric acid-reactive substances) and enhanced the total antioxidant status of plasma. The effects observed in biological models were in general dose- and TPC-dependent; only for protein nitration the relationships were not significant. Furthermore, in cytotoxicity tests, the extracts did not affect the viability of human peripheral blood mononuclear cells (PBMC), and might be regarded as safe. Among extracts, the defatted methanol-water (7:3, v/v) extract and its diethyl ether and ethyl acetate fractions appear to be the most advantageous for biological applications. As compared to the positive controls, activity of the extracts was favorable, which might be attributed to some synergic effects of their constituents. In conclusion, this research proves that the antioxidant and enzyme inhibitory capacity of phenolic fractions should be counted as one of the mechanisms behind the activity of the flowers reported by traditional medicine and demonstrates the potential of the extracts as alternative ingredients for functional products supporting the treatment of oxidative stress-related pathologies cross-linked with inflammatory changes, especially in cardiovascular protection.
Keywords: LC-MS; Prunus spinosa; antioxidants; human plasma; hyaluronidase; lipoxygenase; oxidative stress; polyphenols.
Figures
Similar articles
-
The Effect of Standardised Flower Extracts of Sorbus aucuparia L. on Proinflammatory Enzymes, Multiple Oxidants, and Oxidative/Nitrative Damage of Human Plasma Components In Vitro.Oxid Med Cell Longev. 2019 Feb 4;2019:9746358. doi: 10.1155/2019/9746358. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 30863484 Free PMC article.
-
Polyphenol-Rich Extracts from Cotoneaster Leaves Inhibit Pro-Inflammatory Enzymes and Protect Human Plasma Components against Oxidative Stress In Vitro.Molecules. 2018 Sep 26;23(10):2472. doi: 10.3390/molecules23102472. Molecules. 2018. PMID: 30261655 Free PMC article.
-
Polyphenol-Enriched Extracts of Prunus spinosa Fruits: Anti-Inflammatory and Antioxidant Effects in Human Immune Cells Ex Vivo in Relation to Phytochemical Profile.Molecules. 2022 Mar 4;27(5):1691. doi: 10.3390/molecules27051691. Molecules. 2022. PMID: 35268792 Free PMC article.
-
Blackthorn-A Valuable Source of Phenolic Antioxidants with Potential Health Benefits.Molecules. 2023 Apr 14;28(8):3456. doi: 10.3390/molecules28083456. Molecules. 2023. PMID: 37110690 Free PMC article. Review.
-
Phenolic acids: Natural versatile molecules with promising therapeutic applications.Biotechnol Rep (Amst). 2019 Aug 20;24:e00370. doi: 10.1016/j.btre.2019.e00370. eCollection 2019 Dec. Biotechnol Rep (Amst). 2019. PMID: 31516850 Free PMC article. Review.
Cited by
-
A Comprehensive Review of Molecular Mechanisms, Pharmacokinetics, Toxicology and Plant Sources of Juglanin: Current Landscape and Future Perspectives.Int J Mol Sci. 2024 Sep 25;25(19):10323. doi: 10.3390/ijms251910323. Int J Mol Sci. 2024. PMID: 39408653 Free PMC article. Review.
-
Influence of Ultrasonication and UV-C Processing on the Functional Characteristics and Anticarcinogenic Activity of Blackthorn Vinegar.ACS Omega. 2024 Aug 16;9(34):36699-36709. doi: 10.1021/acsomega.4c05363. eCollection 2024 Aug 27. ACS Omega. 2024. PMID: 39220535 Free PMC article.
-
Flavonol and A-type procyanidin-rich extracts of Prunus spinosa L. flower exhibit anticoagulant activity through direct thrombin inhibition, but do not affect platelet aggregation in vitro.Front Pharmacol. 2023 Nov 27;14:1307373. doi: 10.3389/fphar.2023.1307373. eCollection 2023. Front Pharmacol. 2023. PMID: 38089051 Free PMC article.
-
The Effect of a High-Protein Diet Supplemented with Blackthorn Flower Extract on Polyphenol Bioavailability and Antioxidant Status in the Organs of C57BL/6 Mice.Nutrients. 2023 Sep 20;15(18):4066. doi: 10.3390/nu15184066. Nutrients. 2023. PMID: 37764849 Free PMC article.
-
Nrf2-Mediated Pathway Activated by Prunus spinosa L. (Rosaceae) Fruit Extract: Bioinformatics Analyses and Experimental Validation.Nutrients. 2023 Apr 28;15(9):2132. doi: 10.3390/nu15092132. Nutrients. 2023. PMID: 37432298 Free PMC article.
References
-
- Barros L., Carvalho A. M., Morais J. S., Ferreira I. C. F. R. (2010). Strawberry-tree, blackthorn and rose fruits: detailed characterisation in nutrients and phytochemicals with antioxidant properties. Food Chem. 120, 247–254. 10.1016/j.foodchem.2009.10.016 - DOI
-
- Baydara N. G., Özkanb G., Sağdiçc O. (2004). Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food Control 15, 335–339. 10.1016/S0956-7135(03)00083-5 - DOI
-
- Berger F. (1949). Handbuch der Drogenkunde, 1st Edn. Wien: Maudrich.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

