Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer
- PMID: 29072975
- PMCID: PMC5791845
- DOI: 10.1200/JCO.2017.73.6785
Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer
Abstract
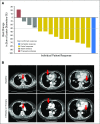
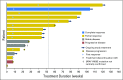
Purpose We report the efficacy and safety of dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) combination therapy in BRAF V600E-mutated anaplastic thyroid cancer, a rare, aggressive, and highly lethal malignancy with poor patient outcomes and no systemic therapies with clinical benefit. Methods In this phase II, open-label trial, patients with predefined BRAF V600E-mutated malignancies received dabrafenib 150 mg twice daily and trametinib 2 mg once daily until unacceptable toxicity, disease progression, or death. The primary end point was investigator-assessed overall response rate. Secondary end points included duration of response, progression-free survival, overall survival, and safety. Results Sixteen patients with BRAF V600E-mutated anaplastic thyroid cancer were evaluable (median follow-up, 47 weeks; range, 4 to 120 weeks). All patients had received prior radiation treatment and/or surgery, and six had received prior systemic therapy. The confirmed overall response rate was 69% (11 of 16; 95% CI, 41% to 89%), with seven ongoing responses. Median duration of response, progression-free survival, and overall survival were not reached as a result of a lack of events, with 12-month estimates of 90%, 79%, and 80%, respectively. The safety population was composed of 100 patients who were enrolled with seven rare tumor histologies. Common adverse events were fatigue (38%), pyrexia (37%), and nausea (35%). No new safety signals were detected. Conclusion Dabrafenib plus trametinib is the first regimen demonstrated to have robust clinical activity in BRAF V600E-mutated anaplastic thyroid cancer and was well tolerated. These findings represent a meaningful therapeutic advance for this orphan disease.
Trial registration: ClinicalTrials.gov NCT02034110.
Figures
Similar articles
-
Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial.Lancet Oncol. 2020 Sep;21(9):1234-1243. doi: 10.1016/S1470-2045(20)30321-1. Epub 2020 Aug 17. Lancet Oncol. 2020. PMID: 32818466 Clinical Trial.
-
Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial.Lancet Oncol. 2017 Jul;18(7):863-873. doi: 10.1016/S1470-2045(17)30429-1. Epub 2017 Jun 4. Lancet Oncol. 2017. PMID: 28592387 Free PMC article. Clinical Trial.
-
Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial.Lancet Oncol. 2017 Oct;18(10):1307-1316. doi: 10.1016/S1470-2045(17)30679-4. Epub 2017 Sep 11. Lancet Oncol. 2017. PMID: 28919011 Clinical Trial.
-
Rechallenge with dabrafenib plus trametinib in anaplastic thyroid cancer: A case report and review of literature.Curr Probl Cancer. 2021 Apr;45(2):100668. doi: 10.1016/j.currproblcancer.2020.100668. Epub 2020 Oct 22. Curr Probl Cancer. 2021. PMID: 33127167 Review.
-
Combination dabrafenib and trametinib in the management of advanced melanoma with BRAFV600 mutations.Expert Opin Pharmacother. 2016;17(7):1031-8. doi: 10.1517/14656566.2016.1168805. Epub 2016 Apr 12. Expert Opin Pharmacother. 2016. PMID: 27027150 Review.
Cited by
-
Dabrafenib and Trametinib in Patients With Tumors With BRAFV600E Mutations: Results of the NCI-MATCH Trial Subprotocol H.J Clin Oncol. 2020 Nov 20;38(33):3895-3904. doi: 10.1200/JCO.20.00762. Epub 2020 Aug 6. J Clin Oncol. 2020. PMID: 32758030 Free PMC article.
-
The Roles of Common Variation and Somatic Mutation in Cancer Pharmacogenomics.Oncol Ther. 2019 Jun;7(1):1-32. doi: 10.1007/s40487-018-0090-6. Epub 2019 Jan 4. Oncol Ther. 2019. PMID: 32700193 Free PMC article. Review.
-
Pembrolizumab in a Patient with Treatment-Naïve Unresectable BRAF-Mutation Negative Anaplastic Thyroid Cancer.Case Rep Endocrinol. 2021 May 22;2021:5521649. doi: 10.1155/2021/5521649. eCollection 2021. Case Rep Endocrinol. 2021. PMID: 34123437 Free PMC article.
-
Clinical cancer genomic profiling.Nat Rev Genet. 2021 Aug;22(8):483-501. doi: 10.1038/s41576-021-00338-8. Epub 2021 Mar 24. Nat Rev Genet. 2021. PMID: 33762738 Review.
-
Surgery combined with adjuvant radiation and chemotherapy prolonged overall survival in stage IVC anaplastic thyroid cancer: a SEER-based analysis.Endocrine. 2024 Jul;85(1):250-257. doi: 10.1007/s12020-023-03662-7. Epub 2024 Jan 6. Endocrine. 2024. PMID: 38183567 Free PMC article.
References
-
- Gilliland FD, Hunt WC, Morris DM, et al. : Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) Program 1973-1991. Cancer 79:564-573, 1997 - PubMed
-
- Kebebew E, Greenspan FS, Clark OH, et al. : Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer 103:1330-1335, 2005 - PubMed
-
- National Cancer Institute : SEER Cancer Stat Facts: Thyroid Cancer. https://seer.cancer.gov/statfacts/html/thyro.html
-
- Lee DY, Won JK, Choi HS, et al. : Recurrence and survival after gross total removal of resectable undifferentiated or poorly differentiated thyroid carcinoma. Thyroid 26:1259-1268, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

