Small Molecule Mediated Restoration of Mitochondrial Function Augments Anti-Mycobacterial Activity of Human Macrophages Subjected to Cholesterol Induced Asymptomatic Dyslipidemia
- PMID: 29067283
- PMCID: PMC5641336
- DOI: 10.3389/fcimb.2017.00439
Small Molecule Mediated Restoration of Mitochondrial Function Augments Anti-Mycobacterial Activity of Human Macrophages Subjected to Cholesterol Induced Asymptomatic Dyslipidemia
Abstract
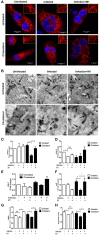
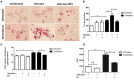
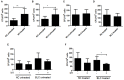
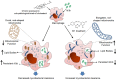
Mycobacterium tuberculosis (M.tb) infection manifests into tuberculosis (TB) in a small fraction of the infected population that comprises the TB susceptible group. Identifying the factors potentiating susceptibility to TB persistence is one of the prime agenda of TB control programs. Recently, WHO recognized diabetes as a risk factor for TB disease progression. The closely related pathological state of metabolic imbalance, dyslipidemia, is yet another emerging risk factor involving deregulation in host immune responses. While high cholesterol levels are clinically proven condition for perturbations in cardiac health, a significant fraction of population these days suffer from borderline risk cholesterol profiles. This apparently healthy population is susceptible to various health risks placing them in the "pre-disease" range. Our study focuses on determining the role of such asymptomatic dyslipidemia as a potential risk factor for susceptibility to TB persistence. Macrophages exposed to sub-pathological levels of cholesterol for chronic period, besides impaired release of TNF-α, could not clear intracellular pathogenic mycobacteria effectively as compared to the unexposed cells. These cells also allowed persistence of opportunistic mycobacterial infection by M. avium and M. bovis BCG, indicating highly compromised immune response. The cholesterol-treated macrophages developed a foamy phenotype with a significant increase in intracellular lipid-bodies prior to M.tb infection, potentially contributing to pre-disease state for tuberculosis infection. The foamy phenotype, known to support M.tb infection, increased several fold upon infection in these cells. Additionally, mitochondrial morphology and function were perturbed, more so during infection in cholesterol treated cells. Pharmacological supplementation with small molecule M1 that restored mitochondrial structural and functional integrity limited M.tb survival more effectively in cholesterol exposed macrophages. Mechanistically, M1 molecule promoted clearance of mycobacteria by reducing total cellular lipid content and restoring mitochondrial morphology and function to its steady state. We further supported our observations by infection assays in PBMC-derived macrophages from clinically healthy volunteers with borderline risk cholesterol profiles. With these observations, we propose that prolonged exposure to sub-pathological cholesterol can lead to asymptomatic susceptibility to M.tb persistence. Use of small molecules like M1 sets yet another strategy for host-directed therapy where re-functioning of mitochondria in cholesterol abused macrophages can improve M.tb clearance.
Keywords: Mycobacterium tuberculosis; cholesterol; dyslipidemia; infection; mitochondria; pre-disease; small molecule M1.
Figures

Similar articles
-
[Frontier of mycobacterium research--host vs. mycobacterium].Kekkaku. 2005 Sep;80(9):613-29. Kekkaku. 2005. PMID: 16245793 Japanese.
-
N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions.BMC Microbiol. 2016 Oct 28;16(1):251. doi: 10.1186/s12866-016-0872-7. BMC Microbiol. 2016. PMID: 27793104 Free PMC article.
-
Delivery of LLKKK18 loaded into self-assembling hyaluronic acid nanogel for tuberculosis treatment.J Control Release. 2016 Aug 10;235:112-124. doi: 10.1016/j.jconrel.2016.05.064. Epub 2016 Jun 1. J Control Release. 2016. PMID: 27261333
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
-
Mycobacterium tuberculosis Infection-Driven Foamy Macrophages and Their Implications in Tuberculosis Control as Targets for Host-Directed Therapy.Front Immunol. 2020 May 12;11:910. doi: 10.3389/fimmu.2020.00910. eCollection 2020. Front Immunol. 2020. PMID: 32477367 Free PMC article. Review.
Cited by
-
Mitochondria: Powering the Innate Immune Response to Mycobacterium tuberculosis Infection.Infect Immun. 2021 Mar 17;89(4):e00687-20. doi: 10.1128/IAI.00687-20. Print 2021 Mar 17. Infect Immun. 2021. PMID: 33558322 Free PMC article. Review.
-
Host-directed therapy to combat mycobacterial infections.Immunol Rev. 2021 May;301(1):62-83. doi: 10.1111/imr.12951. Epub 2021 Feb 9. Immunol Rev. 2021. PMID: 33565103 Free PMC article. Review.
-
Host-Directed Therapies for Tuberculosis.Pathogens. 2022 Nov 3;11(11):1291. doi: 10.3390/pathogens11111291. Pathogens. 2022. PMID: 36365041 Free PMC article. Review.
-
Fatty acid oxidation of alternatively activated macrophages prevents foam cell formation, but Mycobacterium tuberculosis counteracts this process via HIF-1α activation.PLoS Pathog. 2020 Oct 1;16(10):e1008929. doi: 10.1371/journal.ppat.1008929. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33002063 Free PMC article.
-
Mitochondrial dynamics and their potential as a therapeutic target.Mitochondrion. 2019 Nov;49:269-283. doi: 10.1016/j.mito.2019.06.002. Epub 2019 Jun 19. Mitochondrion. 2019. PMID: 31228566 Free PMC article. Review.
References
-
- ATS (2000). Diagnostic standards and classification of tuberculosis in adults and children. this official statement of the American thoracic society and the centers for disease control and prevention was adopted by the ATS board of directors, July 1999. this statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am. J. Respir. Crit. Care Med. 161(4 Pt 1), 1376–1395. 10.1164/ajrccm.161.4.16141 - DOI - PubMed
-
- Almeida P. E., Silva A. R., Maya-Monteiro C. M., Torocsik D., D'Avila H., Dezso B., et al. . (2009). Mycobacterium bovis bacillus Calmette-Guerin infection induces TLR2-dependent peroxisome proliferator-activated receptor gamma expression and activation: functions in inflammation, lipid metabolism, and pathogenesis. J. Immunol. 183, 1337–1345. 10.4049/jimmunol.0900365 - DOI - PubMed
-
- Asalla S., Girada S. B., Kuna R. S., Chowdhury D., Kandagatla B., Oruganti S., et al. . (2016). Restoring mitochondrial function: a small molecule-mediated approach to enhance glucose stimulated insulin secretion in cholesterol accumulated pancreatic beta cells. Sci. Rep. 6:27513. 10.1038/srep27513 - DOI - PMC - PubMed
-
- Beutler B. A. (1999). The role of tumor necrosis factor in health and disease. J. Rheumatol. Suppl. 57, 16–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

