Stem Cell Secretome and Its Effect on Cellular Mechanisms Relevant to Wound Healing
- PMID: 29066165
- PMCID: PMC5835016
- DOI: 10.1016/j.ymthe.2017.09.023
Stem Cell Secretome and Its Effect on Cellular Mechanisms Relevant to Wound Healing
Abstract
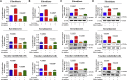
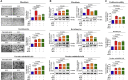
Stem cells introduced to site of injury primarily act via indirect paracrine effects rather than direct cell replacement of damaged cells. This gives rise to understanding the stem cell secretome. In this study, in vitro studies demonstrate that the secretome activates the PI3K/Akt or FAK/ERK1/2 signaling cascades and subsequently enhances the proliferative and migratory abilities of various types of skin cells, such as fibroblasts, keratinocytes, and vascular epithelial cells, ultimately accelerating wound contraction. Indeed, inhibition of these signaling pathways with synthetic inhibitors resulted in the disruption of secretome-induced beneficial effects on various skin cells. In addition, major components of the stem cell secretome (EGF, basic FGF, and HGF) may be responsible for the acceleration of wound contraction. Stimulatory effects of these three prominent factors on wound contraction are achieved through the upregulation of PI3K/Akt or FAK/ERK1/2 activity. Overall, we lay the rationale for using the stem cell secretome in promoting wound contraction. In vivo wound healing studies are warranted to test the significance of our in vitro findings.
Keywords: paracrine effect; stem cell secretome; wound healing.
Copyright © 2017 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Human amniotic epithelial stem cells promote wound healing by facilitating migration and proliferation of keratinocytes via ERK, JNK and AKT signaling pathways.Cell Tissue Res. 2016 Jul;365(1):85-99. doi: 10.1007/s00441-016-2366-1. Epub 2016 Feb 18. Cell Tissue Res. 2016. PMID: 26888423
-
Ozone oil promotes wound healing by increasing the migration of fibroblasts via PI3K/Akt/mTOR signaling pathway.Biosci Rep. 2017 Nov 9;37(6):BSR20170658. doi: 10.1042/BSR20170658. Print 2017 Dec 22. Biosci Rep. 2017. PMID: 28864782 Free PMC article.
-
The stimulatory effects of Stewartia koreana extract on the proliferation and migration of fibroblasts and the wound healing activity of the extract in mice.Int J Mol Med. 2014 Jul;34(1):145-52. doi: 10.3892/ijmm.2014.1753. Epub 2014 Apr 24. Int J Mol Med. 2014. PMID: 24789471
-
Review of the adipose derived stem cell secretome.Biochimie. 2013 Dec;95(12):2222-8. doi: 10.1016/j.biochi.2013.06.001. Epub 2013 Jun 14. Biochimie. 2013. PMID: 23770442 Review.
-
Therapeutic Application of Cell Secretomes in Cutaneous Wound Healing.J Invest Dermatol. 2023 Jun;143(6):893-912. doi: 10.1016/j.jid.2023.02.019. J Invest Dermatol. 2023. PMID: 37211377 Review.
Cited by
-
Topical application of a hyaluronic acid-based hydrogel integrated with secretome of human mesenchymal stromal cells for diabetic ulcer repair.Regen Ther. 2024 Jul 27;26:520-532. doi: 10.1016/j.reth.2024.07.008. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39156755 Free PMC article.
-
State of the field: cellular and exosomal therapeutic approaches in vascular regeneration.Am J Physiol Heart Circ Physiol. 2022 Apr 1;322(4):H647-H680. doi: 10.1152/ajpheart.00674.2021. Epub 2022 Feb 18. Am J Physiol Heart Circ Physiol. 2022. PMID: 35179976 Free PMC article. Review.
-
Secretome of Adipose Tissue-Derived Stem Cells (ASCs) as a Novel Trend in Chronic Non-Healing Wounds: An Overview of Experimental In Vitro and In Vivo Studies and Methodological Variables.Int J Mol Sci. 2019 Jul 30;20(15):3721. doi: 10.3390/ijms20153721. Int J Mol Sci. 2019. PMID: 31366040 Free PMC article. Review.
-
Human Umbilical Cord-Derived Mesenchymal Stem Cells Ameliorate Skin Aging of Nude Mice Through Autophagy-Mediated Anti-Senescent Mechanism.Stem Cell Rev Rep. 2022 Aug;18(6):2088-2103. doi: 10.1007/s12015-022-10418-9. Epub 2022 Jul 21. Stem Cell Rev Rep. 2022. PMID: 35864432
-
Lactobacillus reuteri extracts promoted wound healing via PI3K/AKT/β-catenin/TGFβ1 pathway.Stem Cell Res Ther. 2019 Aug 7;10(1):243. doi: 10.1186/s13287-019-1324-8. Stem Cell Res Ther. 2019. PMID: 31391121 Free PMC article.
References
-
- Song S.H., Lee M.O., Lee J.S., Jeong H.C., Kim H.G., Kim W.S., Hur M., Cha H.J. Genetic modification of human adipose-derived stem cells for promoting wound healing. J. Dermatol. Sci. 2012;66:98–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

