Protective Effect of Akkermansia muciniphila against Immune-Mediated Liver Injury in a Mouse Model
- PMID: 29033903
- PMCID: PMC5626943
- DOI: 10.3389/fmicb.2017.01804
Protective Effect of Akkermansia muciniphila against Immune-Mediated Liver Injury in a Mouse Model
Abstract
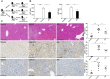
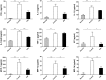
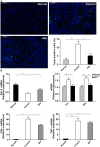
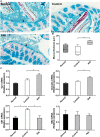
Accumulating evidence indicates that gut microbiota participates in the pathogenesis and progression of liver diseases. The severity of immune-mediated liver injury is associated with different microbial communities. Akkermansia muciniphila can regulate immunologic and metabolic functions. However, little is known about its effects on gut microbiota structure and function. This study investigated the effect of A. muciniphila on immune-mediated liver injury and potential underlying mechanisms. Twenty-two C57BL/6 mice were assigned to three groups (N = 7-8 per group) and continuously administrated A. muciniphila MucT or PBS by oral gavage for 14 days. Mouse feces were collected for gut microbiota analysis on the 15th day, and acute liver injury was induced by Concanavalin A (Con A, 15 mg/kg) injection through the tail vein. Samples (blood, liver, ileum, colon) were assessed for liver injury, systemic inflammation, and intestinal barrier function. We found that oral administration of A. muciniphila decreased serum ALT and AST and alleviated liver histopathological damage induced by Con A. Serum levels of pro-inflammatory cytokines and chemokines (IL-2, IFN-γ, IL-12p40, MCP-1, MIP-1a, MIP-1b) were substantially attenuated. A. muciniphila significantly decreased hepatocellular apoptosis; Bcl-2 expression increased, but Fas and DR5 decreased. Further investigation showed that A. muciniphila enhanced expression of Occludin and Tjp-1 and inhibited CB1 receptor, which strengthened intestinal barriers and reduced systemic LPS level. Fecal 16S rRNA sequence analysis indicated that A. muciniphila increased microbial richness and diversity. The community structure of the Akk group clustered distinctly from that of mice pretreated with PBS. Relative abundance of Firmicutes increased, and Bacteroidetes abundance decreased. Correlation analysis showed that injury-related factors (IL-12p40, IFN-γ, DR5) were negatively associated with specific genera (Ruminococcaceae_UCG_009, Lachnospiraceae_UCG_001, Akkermansia), which were enriched in mice pretreated with A. muciniphila. Our results suggested that A. muciniphila MucT had beneficial effects on immune-mediated liver injury by alleviating inflammation and hepatocellular death. These effects may be driven by the protective profile of the intestinal community induced by the bacteria. The results provide a new perspective on the immune function of gut microbiota in host diseases.
Keywords: Akkermansia muciniphila; acute hepatitis; immune regulation; liver injury; microbiota.
Figures
Similar articles
-
Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice.Front Microbiol. 2019 Oct 1;10:2259. doi: 10.3389/fmicb.2019.02259. eCollection 2019. Front Microbiol. 2019. PMID: 31632373 Free PMC article.
-
The Protective Effects of Live and Pasteurized Akkermansia muciniphila and Its Extracellular Vesicles against HFD/CCl4-Induced Liver Injury.Microbiol Spectr. 2021 Oct 31;9(2):e0048421. doi: 10.1128/Spectrum.00484-21. Epub 2021 Sep 22. Microbiol Spectr. 2021. PMID: 34549998 Free PMC article.
-
Akkermansia muciniphila Ameliorates Acetaminophen-Induced Liver Injury by Regulating Gut Microbial Composition and Metabolism.Microbiol Spectr. 2022 Feb 23;10(1):e0159621. doi: 10.1128/spectrum.01596-21. Epub 2022 Feb 2. Microbiol Spectr. 2022. PMID: 35107323 Free PMC article.
-
The Bacterium Akkermansia muciniphila: A Sentinel for Gut Permeability and Its Relevance to HIV-Related Inflammation.Front Immunol. 2020 Apr 9;11:645. doi: 10.3389/fimmu.2020.00645. eCollection 2020. Front Immunol. 2020. PMID: 32328074 Free PMC article. Review.
-
The metabolic, protective, and immune functions of Akkermansia muciniphila.Microbiol Res. 2023 Jan;266:127245. doi: 10.1016/j.micres.2022.127245. Epub 2022 Oct 28. Microbiol Res. 2023. PMID: 36347103 Review.
Cited by
-
Akkermansia muciniphila MucT attenuates sodium valproate-induced hepatotoxicity and upregulation of Akkermansia muciniphila in rats.J Cell Mol Med. 2024 Jan;28(1):e18026. doi: 10.1111/jcmm.18026. Epub 2023 Nov 14. J Cell Mol Med. 2024. PMID: 37961985 Free PMC article.
-
Preoperative Microbiomes and Intestinal Barrier Function Can Differentiate Prodromal Alzheimer's Disease From Normal Neurocognition in Elderly Patients Scheduled to Undergo Orthopedic Surgery.Front Cell Infect Microbiol. 2021 Mar 29;11:592842. doi: 10.3389/fcimb.2021.592842. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33869072 Free PMC article.
-
Treadmill Exercise Modulates Intestinal Microbes and Suppresses LPS Displacement to Alleviate Neuroinflammation in the Brains of APP/PS1 Mice.Nutrients. 2022 Oct 5;14(19):4134. doi: 10.3390/nu14194134. Nutrients. 2022. PMID: 36235786 Free PMC article.
-
Multi-omics reveals deoxycholic acid modulates bile acid metabolism via the gut microbiota to antagonize carbon tetrachloride-induced chronic liver injury.Gut Microbes. 2024 Jan-Dec;16(1):2323236. doi: 10.1080/19490976.2024.2323236. Epub 2024 Feb 28. Gut Microbes. 2024. PMID: 38416424 Free PMC article.
-
The association between altered intestinal microbiome, impaired systemic and ocular surface immunity, and impaired wound healing response after corneal alkaline-chemical injury in diabetic mice.Front Immunol. 2023 Jan 31;14:1063069. doi: 10.3389/fimmu.2023.1063069. eCollection 2023. Front Immunol. 2023. PMID: 36798135 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

