Methyltransferase G9a promotes cervical cancer angiogenesis and decreases patient survival
- PMID: 28977928
- PMCID: PMC5617488
- DOI: 10.18632/oncotarget.19060
Methyltransferase G9a promotes cervical cancer angiogenesis and decreases patient survival
Abstract
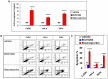
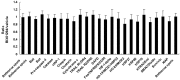
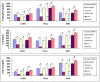
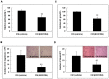
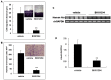
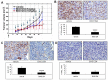
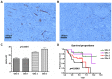
Research suggests that the epigenetic regulator G9a, a H3K9 histone methyltransferase, is involved in cancer invasion and metastasis. Here we show that G9a is linked to cancer angiogenesis and poor patient survival. Invasive cervical cancer has a higher G9a expression than cancer precursors or normal epithelium. Pharmacological inhibition and genetic silencing of G9a suppresses H3K9 methylation, cancer cell proliferation, angiogenesis, and cancer cell invasion/migration, but not apoptosis. Microarray and quantitative reverse transcription polymerase chain reaction analyses reveal that G9a induces a cohort of angiogenic factors that include angiogenin, interleukin-8, and C-X-C motif chemokine ligand 16. Depressing G9a by either pharmacological inhibitor or gene knock down significantly reduces angiogenic factor expression. Moreover, promoting G9a gene expression augments transcription and angiogenic function. A luciferase reporter assay suggests that knockdown of G9a inhibits transcriptional activation of interleukin-8. G9a depletion suppresses xenograft tumor growth in mouse model, which is linked to a decrease in microvessel density and proliferating cell nuclear antigen expression. Clinically, higher G9a expression correlates with poorer survival for cancer patients. For patients' primary tumors a positive correlation between G9a expression and microvessel density also exists. In addition to increasing tumor cell proliferation, G9a promotes tumor angiogenesis and reduces the patient survival rate. G9a may possess great value for targeted therapies.
Keywords: G9a; angiogenesis; cancer cell proliferation; patient survival; xenograft.
Conflict of interest statement
CONFLICTS OF INTEREST None declared.
Figures




Similar articles
-
H3K9 histone methyltransferase G9a promotes lung cancer invasion and metastasis by silencing the cell adhesion molecule Ep-CAM.Cancer Res. 2010 Oct 15;70(20):7830-40. doi: 10.1158/0008-5472.CAN-10-0833. Epub 2010 Oct 12. Cancer Res. 2010. PMID: 20940408
-
Depletion of G9a gene induces cell apoptosis in human gastric carcinoma.Oncol Rep. 2016 May;35(5):3041-9. doi: 10.3892/or.2016.4692. Epub 2016 Mar 17. Oncol Rep. 2016. PMID: 27081761
-
The H3K9 Methyltransferase G9a Represses E-cadherin and is Associated with Myometrial Invasion in Endometrial Cancer.Ann Surg Oncol. 2015 Dec;22 Suppl 3:S1556-65. doi: 10.1245/s10434-015-4379-5. Epub 2015 Jan 23. Ann Surg Oncol. 2015. PMID: 25613390
-
Novel Function of Lysine Methyltransferase G9a in the Regulation of Sox2 Protein Stability.PLoS One. 2015 Oct 22;10(10):e0141118. doi: 10.1371/journal.pone.0141118. eCollection 2015. PLoS One. 2015. PMID: 26492085 Free PMC article.
-
Functional Role of G9a Histone Methyltransferase in Cancer.Front Immunol. 2015 Sep 25;6:487. doi: 10.3389/fimmu.2015.00487. eCollection 2015. Front Immunol. 2015. PMID: 26441991 Free PMC article. Review.
Cited by
-
Epigenetic Regulation of Angiogenesis in Development and Tumors Progression: Potential Implications for Cancer Treatment.Front Cell Dev Biol. 2021 Sep 6;9:689962. doi: 10.3389/fcell.2021.689962. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34552922 Free PMC article. Review.
-
Discovery of a Novel Chemotype of Histone Lysine Methyltransferase EHMT1/2 (GLP/G9a) Inhibitors: Rational Design, Synthesis, Biological Evaluation, and Co-crystal Structure.J Med Chem. 2019 Mar 14;62(5):2666-2689. doi: 10.1021/acs.jmedchem.8b02008. Epub 2019 Feb 26. J Med Chem. 2019. PMID: 30753076 Free PMC article.
-
An Updated Review on the Significance of DNA and Protein Methyltransferases and De-methylases in Human Diseases: From Molecular Mechanism to Novel Therapeutic Approaches.Curr Med Chem. 2024;31(23):3550-3587. doi: 10.2174/0929867330666230607124803. Curr Med Chem. 2024. PMID: 37287285 Review.
-
Chrysin Modulates Aberrant Epigenetic Variations and Hampers Migratory Behavior of Human Cervical (HeLa) Cells.Front Genet. 2022 Jan 12;12:768130. doi: 10.3389/fgene.2021.768130. eCollection 2021. Front Genet. 2022. PMID: 35096000 Free PMC article.
-
SUV39H1 Inhibits Angiogenesis in Limb Ischemia of Mice.Cell Transplant. 2023 Jan-Dec;32:9636897231198167. doi: 10.1177/09636897231198167. Cell Transplant. 2023. PMID: 37811706 Free PMC article.
References
-
- Mathon NF, Lloyd AC. Milestones in cell division: cell senescence and cancer. Nat Rev Cancer. 2001;1:203–213. - PubMed
-
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. - PubMed
-
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. - PubMed
-
- Fidler IJ. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

