Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer
- PMID: 28975219
- PMCID: PMC6583041
- DOI: 10.1001/jamaoncol.2017.2925
Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer
Abstract
Importance: Immune-related adverse events (irAEs) have been associated with the efficacy of PD-1 (programmed cell death protein 1) inhibitors in patients with melanoma, but whether such an association exists for non-small-cell lung cancer (NSCLC) has remained unknown.
Objective: To evaluate the relation of irAEs to nivolumab efficacy in NSCLC.
Design, setting, and participants: In this study based on landmark and multivariable analyses, a total of 134 patients with advanced or recurrent NSCLC who were treated with nivolumab in the second-line setting or later between December 2015 and August 2016 were identified from a review of medical records from multiple institutions, including a university hospital and community hospitals. Data were updated as of December 31, 2016.
Exposures: The absence or presence of any irAE before the landmark date.
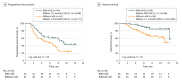
Main outcomes and measures: Kaplan-Meier curves of progression-free survival (PFS) according to the development of irAEs in 6-week landmark analysis were evaluated with the log-rank test as a preplanned primary objective. Overall survival (OS) was similarly evaluated. Multivariable analysis of both PFS and OS was performed with Cox proportional hazard regression models.
Results: In a cohort of 134 patients (median [range] age, 68 [33-85] years; 90 men [67%], 44 women [33%]), irAEs were observed in 69 of the 134 study patients (51%), including 12 patients (9%) with such events of grade 3 or 4, and 24 patients (18%) requiring systemic corticosteroid therapy. In 6-week landmark analysis, median PFS was 9.2 months (95% CI, 4.4 to not reached [NR]) and 4.8 months (95% CI, 3.0 to 7.5) (P = .04) whereas median OS was NR (95% CI, 12.3 to NR) and 11.1 months (95% CI, 9.6 to NR) (P = .01) for patients with or without irAEs, respectively. Multivariable analysis also revealed that irAEs were positively associated with survival outcome, with hazard ratios of 0.525 (95% CI, 0.287 to 0.937; P = .03) for PFS and 0.282 (95% CI, 0.101 to 0.667; P = .003) for OS.
Conclusions and relevance: Development of irAEs was associated with survival outcome of nivolumab treatment in patients with advanced or recurrent NSCLC. Further studies are needed to confirm our findings.
Conflict of interest statement
Figures
Comment in
-
Immune-Related Adverse Events-A Novel Prediction of Nivolumab Efficacy?-Reply.JAMA Oncol. 2018 Jul 1;4(7):1017-1018. doi: 10.1001/jamaoncol.2018.0751. JAMA Oncol. 2018. PMID: 29852041 No abstract available.
-
Immune-Related Adverse Events-A Novel Prediction of Nivolumab Efficacy?JAMA Oncol. 2018 Jul 1;4(7):1017. doi: 10.1001/jamaoncol.2018.0745. JAMA Oncol. 2018. PMID: 29852042 No abstract available.
Similar articles
-
Correlations Between the Immune-related Adverse Events Spectrum and Efficacy of Anti-PD1 Immunotherapy in NSCLC Patients.Clin Lung Cancer. 2019 Jul;20(4):237-247.e1. doi: 10.1016/j.cllc.2019.02.006. Epub 2019 Feb 21. Clin Lung Cancer. 2019. PMID: 30885550
-
Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis.J Cancer Res Clin Oncol. 2019 Feb;145(2):479-485. doi: 10.1007/s00432-018-2805-3. Epub 2018 Dec 1. J Cancer Res Clin Oncol. 2019. PMID: 30506406
-
Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab.Lung Cancer. 2018 Jan;115:71-74. doi: 10.1016/j.lungcan.2017.11.019. Epub 2017 Nov 21. Lung Cancer. 2018. PMID: 29290265
-
Analysis of the association between prospectively collected immune-related adverse events and survival in patients with solid tumor treated with immune-checkpoint blockers, taking into account immortal-time bias.Cancer Treat Rev. 2022 Nov;110:102452. doi: 10.1016/j.ctrv.2022.102452. Epub 2022 Aug 10. Cancer Treat Rev. 2022. PMID: 35998515 Review.
-
Non-specific symptoms as a prodrome of immune-related adverse events in patients with non-small cell lung cancer receiving nivolumab: a consecutive analysis of 200 patients.J Cancer Res Clin Oncol. 2023 Jul;149(7):3185-3191. doi: 10.1007/s00432-022-04205-9. Epub 2022 Jul 28. J Cancer Res Clin Oncol. 2023. PMID: 35900471 Free PMC article. Review.
Cited by
-
Safety and efficacy of retreatment with immune checkpoint inhibitors in non-small cell lung cancer: a systematic review and meta-analysis.Transl Lung Cancer Res. 2022 Aug;11(8):1555-1566. doi: 10.21037/tlcr-22-140. Transl Lung Cancer Res. 2022. PMID: 36090645 Free PMC article.
-
Thromboembolism during immune checkpoint inhibitor therapy: frequency and risk factors.Discov Oncol. 2024 Oct 5;15(1):527. doi: 10.1007/s12672-024-01416-z. Discov Oncol. 2024. PMID: 39367999 Free PMC article.
-
Questionnaire-based detection of immune-related adverse events in cancer patients treated with PD-1/PD-L1 immune checkpoint inhibitors.BMC Cancer. 2021 Mar 24;21(1):314. doi: 10.1186/s12885-021-08006-0. BMC Cancer. 2021. PMID: 33761922 Free PMC article.
-
Identifying Early Predictive Markers for Immune-Related Adverse Events in Nivolumab-Treated Patients with Renal Cell Carcinoma and Gastric Cancer.Asian Pac J Cancer Prev. 2022 Feb 1;23(2):695-701. doi: 10.31557/APJCP.2022.23.2.695. Asian Pac J Cancer Prev. 2022. PMID: 35225483 Free PMC article.
-
Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab.Sci Rep. 2019 Oct 1;9(1):14039. doi: 10.1038/s41598-019-50574-6. Sci Rep. 2019. PMID: 31575933 Free PMC article.
References
-
- Herbst RS, Baas P, Kim D-W, et al. . Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. - PubMed
-
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. - PubMed
-
- Boutros C, Tarhini A, Routier E, et al. . Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473-486. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials