Inflammasome Activation by Bacterial Outer Membrane Vesicles Requires Guanylate Binding Proteins
- PMID: 28974614
- PMCID: PMC5626967
- DOI: 10.1128/mBio.01188-17
Inflammasome Activation by Bacterial Outer Membrane Vesicles Requires Guanylate Binding Proteins
Abstract
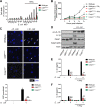
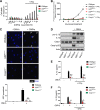
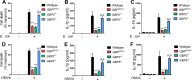
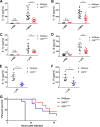
The Gram-negative bacterial cell wall component lipopolysaccharide (LPS) is recognized by the noncanonical inflammasome protein caspase-11 in the cytosol of infected host cells and thereby prompts an inflammatory immune response linked to sepsis. Host guanylate binding proteins (GBPs) promote infection-induced caspase-11 activation in tissue culture models, and yet their in vivo role in LPS-mediated sepsis has remained unexplored. LPS can be released from lysed bacteria as "free" LPS aggregates or actively secreted by live bacteria as a component of outer membrane vesicles (OMVs). Here, we report that GBPs control inflammation and sepsis in mice injected with either free LPS or purified OMVs derived from Gram-negative Escherichia coli In agreement with our observations from in vivo experiments, we demonstrate that macrophages lacking GBP2 expression fail to induce pyroptotic cell death and proinflammatory interleukin-1β (IL-1β) and IL-18 secretion when exposed to OMVs. We propose that in order to activate caspase-11 in vivo, GBPs control the processing of bacterium-derived OMVs by macrophages as well as the processing of circulating free LPS by as-yet-undetermined cell types.IMPORTANCE The bacterial cell wall component LPS is a strong inducer of inflammation and is responsible for much of the toxicity of Gram-negative bacteria. Bacteria shed some of their cell wall and its associated LPS in the form of outer membrane vesicles (OMVs). Recent work demonstrated that secreted OMVs deliver LPS into the host cell cytosol by an unknown mechanism, resulting in the activation of the proinflammatory LPS sensor caspase-11. Here, we show that activation of cytosolic caspase-11 by OMVs requires additional host factors, the so-called guanylate binding proteins (GBPs). The discovery of GBPs as regulators of OMV-mediated inflammation paves the way toward a mechanistic understanding of the host response toward bacterial OMVs and may lead to effective strategies to ameliorate inflammation induced by bacterial infections.
Keywords: GBP2; GBP5; LPS; OMVs; caspase-11; guanylate binding proteins; inflammasome; interferons; lipopolysaccharide; outer membrane vesicles; sepsis.
Copyright © 2017 Finethy et al.
Figures
Similar articles
-
Bacterial Outer Membrane Vesicle-Mediated Cytosolic Delivery of Flagellin Triggers Host NLRC4 Canonical Inflammasome Signaling.Front Immunol. 2020 Nov 18;11:581165. doi: 10.3389/fimmu.2020.581165. eCollection 2020. Front Immunol. 2020. PMID: 33312172 Free PMC article.
-
LPS-aggregating proteins GBP1 and GBP2 are each sufficient to enhance caspase-4 activation both in cellulo and in vitro.Proc Natl Acad Sci U S A. 2023 Apr 11;120(15):e2216028120. doi: 10.1073/pnas.2216028120. Epub 2023 Apr 6. Proc Natl Acad Sci U S A. 2023. PMID: 37023136 Free PMC article.
-
LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation.EMBO J. 2018 Mar 15;37(6):e98089. doi: 10.15252/embj.201798089. Epub 2018 Feb 19. EMBO J. 2018. PMID: 29459437 Free PMC article.
-
Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses.Immunology. 2017 Oct;152(2):207-217. doi: 10.1111/imm.12787. Epub 2017 Jul 31. Immunology. 2017. PMID: 28695629 Free PMC article. Review.
-
Lipopolysaccharide Recognition in the Crossroads of TLR4 and Caspase-4/11 Mediated Inflammatory Pathways.Front Immunol. 2020 Nov 27;11:585146. doi: 10.3389/fimmu.2020.585146. eCollection 2020. Front Immunol. 2020. PMID: 33329561 Free PMC article. Review.
Cited by
-
C57BL/6 and 129 inbred mouse strains differ in Gbp2 and Gbp2b expression in response to inflammatory stimuli in vivo.Wellcome Open Res. 2019 Aug 20;4:124. doi: 10.12688/wellcomeopenres.15329.1. eCollection 2019. Wellcome Open Res. 2019. PMID: 31544161 Free PMC article.
-
SNX10-mediated LPS sensing causes intestinal barrier dysfunction via a caspase-5-dependent signaling cascade.EMBO J. 2021 Dec 15;40(24):e108080. doi: 10.15252/embj.2021108080. Epub 2021 Nov 8. EMBO J. 2021. PMID: 34747049 Free PMC article.
-
The extracellular vesicle generation paradox: a bacterial point of view.EMBO J. 2021 Nov 2;40(21):e108174. doi: 10.15252/embj.2021108174. Epub 2021 Oct 11. EMBO J. 2021. PMID: 34636061 Free PMC article. Review.
-
Human GBP1 is a microbe-specific gatekeeper of macrophage apoptosis and pyroptosis.EMBO J. 2019 Jul 1;38(13):e100926. doi: 10.15252/embj.2018100926. Epub 2019 Jun 3. EMBO J. 2019. PMID: 31268602 Free PMC article.
-
Caspase-11-mediated enteric neuronal pyroptosis underlies Western diet-induced colonic dysmotility.J Clin Invest. 2020 Jul 1;130(7):3621-3636. doi: 10.1172/JCI130176. J Clin Invest. 2020. PMID: 32484462 Free PMC article.
References
-
- Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszyński A, Forsberg LS, Carlson RW, Dixit VM. 2013. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341:1246–1249. doi:10.1126/science.1240248. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
