Cinnamomum zeylanicum (Ceylon cinnamon) as a potential pharmaceutical agent for type-2 diabetes mellitus: study protocol for a randomized controlled trial
- PMID: 28962661
- PMCID: PMC5622575
- DOI: 10.1186/s13063-017-2192-0
Cinnamomum zeylanicum (Ceylon cinnamon) as a potential pharmaceutical agent for type-2 diabetes mellitus: study protocol for a randomized controlled trial
Abstract
Background: Previous studies have explored the anti-diabetic effects of Cinnamomum cassia extract in vivo and in vitro. However, there are no studies at present exploring the effects of the indigenous species of Sri Lankan cinnamon (Cinnamomum zeylanicum) in patients with diabetes mellitus. The present study aims to evaluate the potential effects of Cinnamomum zeylanicum extract as a pharmaceutical agent in patients with type-2 diabetes mellitus.
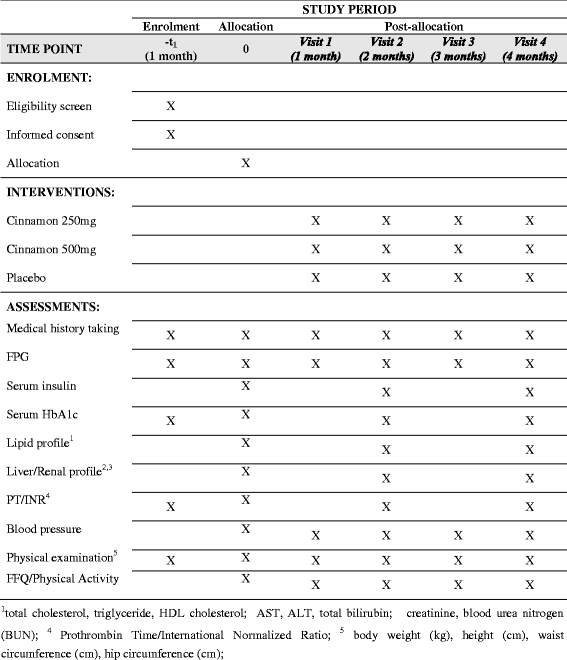
Methods/design: The study will be conducted as a randomized, double-blind, placebo-controlled clinical trial for a period of 4 months at the Medical Clinic, University Medical Unit, National Hospital of Sri Lanka. A total of 210 subjects with diabetes, in three equal groups, will be recruited for the study. The patients will be randomized in a 1:1:1 ratio according to the method of block randomization and the subjects will be randomly and equally assigned into two test groups (n = 70 each) and one placebo group (n = 70). The population will be stratified at randomization based on age, gender and disease severity. The treatment drug is a capsule containing Cinnamomum zeylanicum extract as the active ingredient and the placebo capsule will contain lactose monohydrate. Two doses of Cinnamomum zeylanicum extracts (250 mg and 500 mg of the cinnamon extract) will be used. The study drugs will be double blinded to both investigators and participants. The visits and the evaluations will be done as follows: screening (visit 0), 1 month (visit 1), 2 months (visit 2), 3 months (visit 3) and 4 months (visit 4). The following primary outcome measures will be evaluated: glycosylated hemoglobin (HbA1c), fasting plasma glucose (FPG) and serum insulin. Secondary outcome measures include: Body Mass Index (BMI) and other anthropometric parameters, blood pressure, total cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL) and triglycerides (TAG). Data will be analyzed using SPSS version 14.
Discussion: We describe the protocol for a clinical trial design evaluating the effects of Cinnamomum zeylanicum (Ceylon cinnamon) in patients with type-2 diabetes mellitus. The result of the present study, positive or negative, should provide a step change in the evidence guiding current and future policies regarding the use of cinnamon dietary supplementation in patients with diabetes.
Trial registration: Sri Lanka Clinical Trials Registry (SLCTR), identifier: SLCTR/2017/010 ( http://slctr.lk/trials/714 ). Registered on 5 April 2017; study protocol version 3.1 21 March 2017.
Keywords: Adults; Ceylon cinnamon; Cinnamomum zeylanicum; Diabetes; Sri Lanka.
Conflict of interest statement
Ethics approval and consent to participate
Study is approved by the Ethics Review Committee, Faculty of Medicine, University of Colombo.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Zinc supplementation in pre-diabetes: study protocol for a randomized controlled trial.Trials. 2013 Feb 19;14:52. doi: 10.1186/1745-6215-14-52. Trials. 2013. PMID: 23421759 Free PMC article. Clinical Trial.
-
Evaluation of pharmacodynamic properties and safety of Cinnamomum zeylanicum (Ceylon cinnamon) in healthy adults: a phase I clinical trial.BMC Complement Altern Med. 2017 Dec 28;17(1):550. doi: 10.1186/s12906-017-2067-7. BMC Complement Altern Med. 2017. PMID: 29282046 Free PMC article. Clinical Trial.
-
Efficacy of cinnamon in patients with type II diabetes mellitus: A randomized controlled clinical trial.Clin Nutr. 2019 Apr;38(2):549-556. doi: 10.1016/j.clnu.2018.03.003. Epub 2018 Mar 11. Clin Nutr. 2019. PMID: 29605574 Clinical Trial.
-
Do Cinnamon Supplements Have a Role in Glycemic Control in Type 2 Diabetes? A Narrative Review.J Acad Nutr Diet. 2016 Nov;116(11):1794-1802. doi: 10.1016/j.jand.2016.07.015. Epub 2016 Sep 8. J Acad Nutr Diet. 2016. PMID: 27618575 Free PMC article. Review.
-
To what extent does cinnamon administration improve the glycemic and lipid profiles?Clin Nutr ESPEN. 2018 Oct;27:1-9. doi: 10.1016/j.clnesp.2018.07.011. Epub 2018 Aug 13. Clin Nutr ESPEN. 2018. PMID: 30144878 Review.
Cited by
-
Effects of Cinnamon (Cinnamomum zeylanicum) Extract on Adipocyte Differentiation in 3T3-L1 Cells and Lipid Accumulation in Mice Fed a High-Fat Diet.Nutrients. 2023 Dec 14;15(24):5110. doi: 10.3390/nu15245110. Nutrients. 2023. PMID: 38140369 Free PMC article.
-
Diversity of Fungal Communities on Diseased and Healthy Cinnamomum burmannii Fruits and Antibacterial Activity of Secondary Metabolites.Microbiol Spectr. 2023 Jun 15;11(3):e0008023. doi: 10.1128/spectrum.00080-23. Epub 2023 May 10. Microbiol Spectr. 2023. PMID: 37162357 Free PMC article.
-
Essential Oil Chemotypes and Genetic Variability of Cinnamomum verum Leaf Samples Commercialized and Cultivated in the Amazon.Molecules. 2022 Oct 28;27(21):7337. doi: 10.3390/molecules27217337. Molecules. 2022. PMID: 36364159 Free PMC article.
-
Cinnamomum verum J. Presl Bark Contains High Contents of Nicotinamide Mononucleotide.Molecules. 2022 Oct 19;27(20):7054. doi: 10.3390/molecules27207054. Molecules. 2022. PMID: 36296647 Free PMC article.
-
Comparative Metabolite Profiling and Fingerprinting of Medicinal Cinnamon Bark and Its Commercial Preparations via a Multiplex Approach of GC-MS, UV, and NMR Techniques.Metabolites. 2022 Jul 1;12(7):614. doi: 10.3390/metabo12070614. Metabolites. 2022. PMID: 35888738 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous