In vivo activation of latent HIV with a synthetic bryostatin analog effects both latent cell "kick" and "kill" in strategy for virus eradication
- PMID: 28934369
- PMCID: PMC5608406
- DOI: 10.1371/journal.ppat.1006575
In vivo activation of latent HIV with a synthetic bryostatin analog effects both latent cell "kick" and "kill" in strategy for virus eradication
Abstract
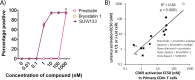
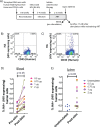
The ability of HIV to establish a long-lived latent infection within resting CD4+ T cells leads to persistence and episodic resupply of the virus in patients treated with antiretroviral therapy (ART), thereby preventing eradication of the disease. Protein kinase C (PKC) modulators such as bryostatin 1 can activate these latently infected cells, potentially leading to their elimination by virus-mediated cytopathic effects, the host's immune response and/or therapeutic strategies targeting cells actively expressing virus. While research in this area has focused heavily on naturally-occurring PKC modulators, their study has been hampered by their limited and variable availability, and equally significantly by sub-optimal activity and in vivo tolerability. Here we show that a designed, synthetically-accessible analog of bryostatin 1 is better-tolerated in vivo when compared with the naturally-occurring product and potently induces HIV expression from latency in humanized BLT mice, a proven and important model for studying HIV persistence and pathogenesis in vivo. Importantly, this induction of virus expression causes some of the newly HIV-expressing cells to die. Thus, designed, synthetically-accessible, tunable, and efficacious bryostatin analogs can mediate both a "kick" and "kill" response in latently-infected cells and exhibit improved tolerability, therefore showing unique promise as clinical adjuvants for HIV eradication.
Conflict of interest statement
I have read the journal’s policy and the authors of the manuscript have the following competing interests: Neurotrope Biosciences and BryoLogyx have licensed some of this technology from Stanford University (P.A.W.), the former for the treatment of neurological disorders such as Alzheimer's disease and the latter for use in HIV/AIDS eradication and cancer immunotherapy. P.A.W is a consultant to both companies and a co-founder of BryoLogyx.
Figures


Similar articles
-
Characterization of designed, synthetically accessible bryostatin analog HIV latency reversing agents.Virology. 2018 Jul;520:83-93. doi: 10.1016/j.virol.2018.05.006. Epub 2018 May 26. Virology. 2018. PMID: 29800728 Free PMC article.
-
Bryostatin-1 Decreases HIV-1 Infection and Viral Production in Human Primary Macrophages.J Virol. 2022 Feb 23;96(4):e0195321. doi: 10.1128/JVI.01953-21. Epub 2021 Dec 8. J Virol. 2022. PMID: 34878918 Free PMC article.
-
An In-Depth Comparison of Latency-Reversing Agent Combinations in Various In Vitro and Ex Vivo HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression.PLoS Pathog. 2015 Jul 30;11(7):e1005063. doi: 10.1371/journal.ppat.1005063. eCollection 2015 Jul. PLoS Pathog. 2015. PMID: 26225566 Free PMC article.
-
Getting the "Kill" into "Shock and Kill": Strategies to Eliminate Latent HIV.Cell Host Microbe. 2018 Jan 10;23(1):14-26. doi: 10.1016/j.chom.2017.12.004. Cell Host Microbe. 2018. PMID: 29324227 Free PMC article. Review.
-
Which therapeutic strategy will achieve a cure for HIV-1?Curr Opin Virol. 2016 Jun;18:14-9. doi: 10.1016/j.coviro.2016.02.001. Epub 2016 Mar 15. Curr Opin Virol. 2016. PMID: 26985878 Review.
Cited by
-
Non-thermal plasma modulates cellular markers associated with immunogenicity in a model of latent HIV-1 infection.PLoS One. 2021 Mar 1;16(3):e0247125. doi: 10.1371/journal.pone.0247125. eCollection 2021. PLoS One. 2021. PMID: 33647028 Free PMC article.
-
Soft Heteroleptic N-Heterocyclic Carbene Palladium(II) Species for Efficient Catalytic Routes to Alkynones via Carbonylative Sonogashira Coupling.ACS Omega. 2020 Aug 18;5(37):23687-23702. doi: 10.1021/acsomega.0c02413. eCollection 2020 Sep 22. ACS Omega. 2020. PMID: 32984688 Free PMC article.
-
Impact of IL-15 and latency reversing agent combinations in the reactivation and NK cell-mediated suppression of the HIV reservoir.Sci Rep. 2022 Nov 3;12(1):18567. doi: 10.1038/s41598-022-23010-5. Sci Rep. 2022. PMID: 36329160 Free PMC article.
-
Designed PKC-targeting bryostatin analogs modulate innate immunity and neuroinflammation.Cell Chem Biol. 2021 Apr 15;28(4):537-545.e4. doi: 10.1016/j.chembiol.2020.12.015. Epub 2021 Jan 19. Cell Chem Biol. 2021. PMID: 33472023 Free PMC article.
-
Pharmacological Activation of Non-canonical NF-κB Signaling Activates Latent HIV-1 Reservoirs In Vivo.Cell Rep Med. 2020 Jun 23;1(3):100037. doi: 10.1016/j.xcrm.2020.100037. eCollection 2020 Jun 23. Cell Rep Med. 2020. PMID: 33205060 Free PMC article.
References
-
- UNAIDS (2016) UNAIDS 2016 Global Fact Sheet.
-
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, et al. (1999) Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 5: 512–517. doi: 10.1038/8394 - DOI - PubMed
-
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, et al. (1997) Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278: 1291–1295. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

