Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial
- PMID: 28916367
- PMCID: PMC5901715
- DOI: 10.1016/S0140-6736(17)32440-6
Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial
Erratum in
-
Department of Error.Lancet. 2017 Oct 28;390(10106):1948. doi: 10.1016/S0140-6736(17)32702-2. Epub 2017 Oct 26. Lancet. 2017. PMID: 29115226 Free PMC article. No abstract available.
Abstract
Background: Rucaparib, a poly(ADP-ribose) polymerase inhibitor, has anticancer activity in recurrent ovarian carcinoma harbouring a BRCA mutation or high percentage of genome-wide loss of heterozygosity. In this trial we assessed rucaparib versus placebo after response to second-line or later platinum-based chemotherapy in patients with high-grade, recurrent, platinum-sensitive ovarian carcinoma.
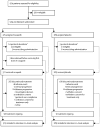
Methods: In this randomised, double-blind, placebo-controlled, phase 3 trial, we recruited patients from 87 hospitals and cancer centres across 11 countries. Eligible patients were aged 18 years or older, had a platinum-sensitive, high-grade serous or endometrioid ovarian, primary peritoneal, or fallopian tube carcinoma, had received at least two previous platinum-based chemotherapy regimens, had achieved complete or partial response to their last platinum-based regimen, had a cancer antigen 125 concentration of less than the upper limit of normal, had a performance status of 0-1, and had adequate organ function. Patients were ineligible if they had symptomatic or untreated central nervous system metastases, had received anticancer therapy 14 days or fewer before starting the study, or had received previous treatment with a poly(ADP-ribose) polymerase inhibitor. We randomly allocated patients 2:1 to receive oral rucaparib 600 mg twice daily or placebo in 28 day cycles using a computer-generated sequence (block size of six, stratified by homologous recombination repair gene mutation status, progression-free interval after the penultimate platinum-based regimen, and best response to the most recent platinum-based regimen). Patients, investigators, site staff, assessors, and the funder were masked to assignments. The primary outcome was investigator-assessed progression-free survival evaluated with use of an ordered step-down procedure for three nested cohorts: patients with BRCA mutations (carcinoma associated with deleterious germline or somatic BRCA mutations), patients with homologous recombination deficiencies (BRCA mutant or BRCA wild-type and high loss of heterozygosity), and the intention-to-treat population, assessed at screening and every 12 weeks thereafter. This trial is registered with ClinicalTrials.gov, number NCT01968213; enrolment is complete.
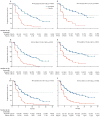
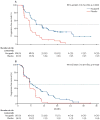
Findings: Between April 7, 2014, and July 19, 2016, we randomly allocated 564 patients: 375 (66%) to rucaparib and 189 (34%) to placebo. Median progression-free survival in patients with a BRCA-mutant carcinoma was 16·6 months (95% CI 13·4-22·9; 130 [35%] patients) in the rucaparib group versus 5·4 months (3·4-6·7; 66 [35%] patients) in the placebo group (hazard ratio 0·23 [95% CI 0·16-0·34]; p<0·0001). In patients with a homologous recombination deficient carcinoma (236 [63%] vs 118 [62%]), it was 13·6 months (10·9-16·2) versus 5·4 months (5·1-5·6; 0·32 [0·24-0·42]; p<0·0001). In the intention-to-treat population, it was 10·8 months (8·3-11·4) versus 5·4 months (5·3-5·5; 0·36 [0·30-0·45]; p<0·0001). Treatment-emergent adverse events of grade 3 or higher in the safety population (372 [99%] patients in the rucaparib group vs 189 [100%] in the placebo group) were reported in 209 (56%) patients in the rucaparib group versus 28 (15%) in the placebo group, the most common of which were anaemia or decreased haemoglobin concentration (70 [19%] vs one [1%]) and increased alanine or aspartate aminotransferase concentration (39 [10%] vs none).
Interpretation: Across all primary analysis groups, rucaparib significantly improved progression-free survival in patients with platinum-sensitive ovarian cancer who had achieved a response to platinum-based chemotherapy. ARIEL3 provides further evidence that use of a poly(ADP-ribose) polymerase inhibitor in the maintenance treatment setting versus placebo could be considered a new standard of care for women with platinum-sensitive ovarian cancer following a complete or partial response to second-line or later platinum-based chemotherapy.
Funding: Clovis Oncology.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
RLC reports grants from AstraZeneca, Roche/Genentech, Janssen, OncoMed, Millennium, Merck, Clovis Oncology, Esperance, and AbbVie and reports serving as an advisor to AstraZeneca, Roche/Genentech, Janssen, OncoMed, Millennium, Merck, Clovis Oncology, Esperance, Tesaro, GamaMabs, Pfizer, Genmab, Gradalis, Bayer, and AbbVie. AMO has served on advisory boards for Amgen, Verastem, Clovis Oncology, and Immunovaccine; received support for travel or accommodation from AstraZeneca; and received honoraria from WebRx. DL has served in a consulting or advisory role for AstraZeneca, Clovis Oncology, Roche, Tesaro, and PharmaMar and received support for travel or accommodation from Roche and PharmaMar. CA served on a steering committee for Mateon Therapeutics and has served on advisory boards for Clovis Oncology, Cerulean Pharma, Bayer, VentiRx, and AstraZeneca. AO has served on advisory boards for Roche, AstraZeneca, PharmaMar, Clovis Oncology, and Tesaro and received support for travel or accommodation from Roche, AstraZeneca, and PharmaMar. NC has served in a consulting or advisory role for Roche, AstraZeneca, Tesaro, PharmaMar, Clovis Oncology, and Advaxis. JIW has received research support from AbbVie and AstraZeneca and served on advisory boards for AstraZeneca. AC has served on advisory boards for AstraZeneca and Roche and received research support from AstraZeneca. AL has served on an advisory board for Clovis Oncology, Pfizer, and PharmaMar; reports institutional research grant support from GamaMabs and Merus; and reports boarding and travel expenses for congress activities from AstraZeneca. RWH has served on a speakers bureau for AstraZeneca, Clovis Oncology, and Tesaro. PCF has served on advisory boards for Clovis Oncology and AstraZeneca and received honoraria from AstraZeneca. JCG has served on advisory boards for Roche, AstraZeneca, Janssen, Merck, and Bristol-Myers Squibb and received support for travel or accommodation from Roche, Bristol-Myers Squibb, and Astellas. DMO received research funding from Clovis Oncology; received institutional research support from Amgen, VentiRx, Regeneron, Immunogen, Array Biopharma, Janssen, Clovis Oncology, EMD Serono, Ergomed, Ajinomoto, and Genentech/Roche; served on an advisory board for Clovis Oncology, AstraZeneca, Janssen, Genentech/Roche, Eisai, Tesaro, and Novocure; served on steering committees for Amgen, Tesaro, and Novocure; and served as a consultant to Tesaro and Novocure. JG-D has received research funding from AstraZeneca and served on advisory boards for Janssen, Clovis Oncology, and Genentech/Roche. AF has served on advisory boards for AstraZeneca, Roche, and Tesaro. IAM has served on advisory boards for Clovis Oncology, Tesaro, and AstraZeneca. CLS has served in a consulting or advisory role for AstraZeneca, Clovis Oncology, Roche, and Eisai Australia; received support for travel or accommodation from AstraZeneca, Clovis Oncology, and Roche; and received drugs for research from Eisai Australia; and her institution received in kind research support for parallel laboratory work using rucaparib. TC, LM, JI, SG, CG, TCH, KKL, and HG are employees of Clovis Oncology and MR was employed at Clovis Oncology at the time of the study and owns stock in the company. JS is an employee of Foundation Medicine, the developer of the homologous recombination deficiency assay used in this trial. JAL has served in an advisory role for Clovis Oncology and AstraZeneca and served on a speakers bureau for and received research grants from AstraZeneca. All other authors declare no competing interests.
Figures


Similar articles
-
Rucaparib for patients with platinum-sensitive, recurrent ovarian carcinoma (ARIEL3): post-progression outcomes and updated safety results from a randomised, placebo-controlled, phase 3 trial.Lancet Oncol. 2020 May;21(5):710-722. doi: 10.1016/S1470-2045(20)30061-9. Lancet Oncol. 2020. PMID: 32359490 Free PMC article.
-
Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial.Lancet Oncol. 2017 Jan;18(1):75-87. doi: 10.1016/S1470-2045(16)30559-9. Epub 2016 Nov 29. Lancet Oncol. 2017. PMID: 27908594 Clinical Trial.
-
Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): an international, open-label, randomised, phase 3 trial.Lancet Oncol. 2022 Apr;23(4):465-478. doi: 10.1016/S1470-2045(22)00122-X. Epub 2022 Mar 14. Lancet Oncol. 2022. PMID: 35298906 Clinical Trial.
-
Poly(ADP-ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer.Cochrane Database Syst Rev. 2022 Feb 16;2(2):CD007929. doi: 10.1002/14651858.CD007929.pub4. Cochrane Database Syst Rev. 2022. PMID: 35170751 Free PMC article. Review.
-
Rucaparib: A Review in Ovarian Cancer.Target Oncol. 2019 Apr;14(2):237-246. doi: 10.1007/s11523-019-00629-5. Target Oncol. 2019. PMID: 30830551 Review.
Cited by
-
Efficacy and safety of rucaparib in previously treated, locally advanced or metastatic urothelial carcinoma from a phase 2, open-label trial (ATLAS).BMC Cancer. 2021 May 24;21(1):593. doi: 10.1186/s12885-021-08085-z. BMC Cancer. 2021. PMID: 34030643 Free PMC article. Clinical Trial.
-
A pilot study investigating feasibility of mainstreaming germline BRCA1 and BRCA2 testing in high-risk patients with breast and/or ovarian cancer in three tertiary Cancer Centres in Ireland.Fam Cancer. 2023 Apr;22(2):135-149. doi: 10.1007/s10689-022-00313-0. Epub 2022 Aug 27. Fam Cancer. 2023. PMID: 36029389
-
Real-World Study of Adding Bevacizumab to Chemotherapy for Ovarian, Tubal, and Peritoneal Cancer as Front-Line or Relapse Therapy (ROBOT): 8-Year Experience.Front Oncol. 2020 Jul 14;10:1095. doi: 10.3389/fonc.2020.01095. eCollection 2020. Front Oncol. 2020. PMID: 32760668 Free PMC article.
-
ARRDC3 as a Diagnostic and Prognostic Biomarker for Epithelial Ovarian Cancer Based on Data Mining.Int J Gen Med. 2021 Mar 22;14:967-981. doi: 10.2147/IJGM.S302012. eCollection 2021. Int J Gen Med. 2021. PMID: 33790626 Free PMC article.
-
Clinical and molecular characteristics of ARIEL3 patients who derived exceptional benefit from rucaparib maintenance treatment for high-grade ovarian carcinoma.Gynecol Oncol. 2022 Dec;167(3):404-413. doi: 10.1016/j.ygyno.2022.08.021. Epub 2022 Oct 20. Gynecol Oncol. 2022. PMID: 36273926 Free PMC article. Clinical Trial.
References
-
- International Agency for Reasearch on Cancer. [accessed Aug 1, 2017];GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/summary_table_pop_sel.aspx.
-
- McMeekin DS, Tillmanns T, Chaudry T, et al. Timing isn’t everything: an analysis of when to start salvage chemotherapy in ovarian cancer. Gynecol Oncol. 2004;95:157–164. - PubMed
-
- Herzog TJ, Holloway RW, Stuart GCE. Workshop: options for therapy in ovarian cancer. Gynecol Oncol. 2003;90:S45–S50. - PubMed
-
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–45. - PMC - PubMed
-
- Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

