Versican is produced by Trif- and type I interferon-dependent signaling in macrophages and contributes to fine control of innate immunity in lungs
- PMID: 28912382
- PMCID: PMC5814701
- DOI: 10.1152/ajplung.00353.2017
Versican is produced by Trif- and type I interferon-dependent signaling in macrophages and contributes to fine control of innate immunity in lungs
Abstract
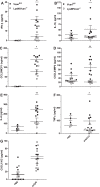
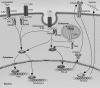
Growing evidence suggests that versican is important in the innate immune response to lung infection. Our goal was to understand the regulation of macrophage-derived versican and the role it plays in innate immunity. We first defined the signaling events that regulate versican expression, using bone marrow-derived macrophages (BMDMs) from mice lacking specific Toll-like receptors (TLRs), TLR adaptor molecules, or the type I interferon receptor (IFNAR1). We show that LPS and polyinosinic-polycytidylic acid [poly(I:C)] trigger a signaling cascade involving TLR3 or TLR4, the Trif adaptor, type I interferons, and IFNAR1, leading to increased expression of versican by macrophages and implicating versican as an interferon-stimulated gene. The signaling events regulating versican are distinct from those for hyaluronan synthase 1 (HAS1) and syndecan-4 in macrophages. HAS1 expression requires TLR2 and MyD88. Syndecan-4 requires TLR2, TLR3, or TLR4 and both MyD88 and Trif. Neither HAS1 nor syndecan-4 is dependent on type I interferons. The importance of macrophage-derived versican in lungs was determined with LysM/Vcan-/- mice. These studies show increased recovery of inflammatory cells in the bronchoalveolar lavage fluid of poly(I:C)-treated LysM/Vcan-/- mice compared with control mice. IFN-β and IL-10, two important anti-inflammatory molecules, are significantly decreased in both poly(I:C)-treated BMDMs from LysM/Vcan-/- mice and bronchoalveolar lavage fluid from poly(I:C)-treated LysM/Vcan-/- mice compared with control mice. In short, type I interferon signaling regulates versican expression, and versican is necessary for type I interferon production. These findings suggest that macrophage-derived versican is an immunomodulatory molecule with anti-inflammatory properties in acute pulmonary inflammation.
Keywords: hyaluronan synthase 1; inflammation; type I interferons; versican, syndecan-4.
Copyright © 2017 the American Physiological Society.
Figures









Similar articles
-
Regulation of versican expression in macrophages is mediated by canonical type I interferon signaling via ISGF3.Am J Physiol Cell Physiol. 2024 Nov 1;327(5):C1274-C1288. doi: 10.1152/ajpcell.00174.2024. Epub 2024 Oct 14. Am J Physiol Cell Physiol. 2024. PMID: 39400584 Free PMC article.
-
A rapid increase in macrophage-derived versican and hyaluronan in infectious lung disease.Matrix Biol. 2014 Feb;34:1-12. doi: 10.1016/j.matbio.2014.01.011. Epub 2014 Jan 26. Matrix Biol. 2014. PMID: 24472738 Free PMC article.
-
A Population of Radio-Resistant Macrophages in the Deep Myenteric Plexus Contributes to Postoperative Ileus Via Toll-Like Receptor 3 Signaling.Front Immunol. 2021 Jan 13;11:581111. doi: 10.3389/fimmu.2020.581111. eCollection 2020. Front Immunol. 2021. PMID: 33519804 Free PMC article.
-
LPS, dsRNA and the interferon bridge to adaptive immune responses: Trif, Tram, and other TIR adaptor proteins.J Endotoxin Res. 2004;10(2):130-6. doi: 10.1179/096805104225004031. J Endotoxin Res. 2004. PMID: 15120005 Review.
-
Versican-A Critical Extracellular Matrix Regulator of Immunity and Inflammation.Front Immunol. 2020 Mar 24;11:512. doi: 10.3389/fimmu.2020.00512. eCollection 2020. Front Immunol. 2020. PMID: 32265939 Free PMC article. Review.
Cited by
-
Long non-coding RNAs (lncRNAs) NEAT1 and MALAT1 are differentially expressed in severe COVID-19 patients: An integrated single-cell analysis.PLoS One. 2022 Jan 10;17(1):e0261242. doi: 10.1371/journal.pone.0261242. eCollection 2022. PLoS One. 2022. PMID: 35007307 Free PMC article.
-
SpaRx: elucidate single-cell spatial heterogeneity of drug responses for personalized treatment.Brief Bioinform. 2023 Sep 22;24(6):bbad338. doi: 10.1093/bib/bbad338. Brief Bioinform. 2023. PMID: 37798249 Free PMC article.
-
ADAMTS proteases in cardiovascular physiology and disease.Open Biol. 2020 Dec;10(12):200333. doi: 10.1098/rsob.200333. Epub 2020 Dec 23. Open Biol. 2020. PMID: 33352066 Free PMC article. Review.
-
Stromal-immune cell crosstalk fundamentally alters the lung microenvironment following tissue insult.Immunology. 2021 Jul;163(3):239-249. doi: 10.1111/imm.13319. Epub 2021 Mar 11. Immunology. 2021. PMID: 33556186 Free PMC article. Review.
-
VUp-Regulation of VCAN Promotes the Proliferation, Invasion and Migration and Serves as a Biomarker in Gastric Cancer.Onco Targets Ther. 2020 Aug 25;13:8665-8675. doi: 10.2147/OTT.S262613. eCollection 2020. Onco Targets Ther. 2020. PMID: 32922041 Free PMC article.
References
-
- Aksoy E, Taboubi S, Torres D, Delbauve S, Hachani A, Whitehead MA, Pearce WP, Berenjeno IM, Nock G, Filloux A, Beyaert R, Flamand V, Vanhaesebroeck B. The p110δ isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat Immunol 13: 1045–1054, 2012. (Erratum. Nat Immunol 14: 877, 2013). doi:10.1038/ni.2426. - DOI - PMC - PubMed
-
- Aksoy E, Vanden Berghe W, Detienne S, Amraoui Z, Fitzgerald KA, Haegeman G, Goldman M, Willems F. Inhibition of phosphoinositide 3-kinase enhances TRIF-dependent NF-kappa B activation and IFN-beta synthesis downstream of Toll-like receptor 3 and 4. Eur J Immunol 35: 2200–2209, 2005. doi:10.1002/eji.200425801. - DOI - PubMed
-
- Andersson-Sjöland A, Hallgren O, Rolandsson S, Weitoft M, Tykesson E, Larsson-Callerfelt AK, Rydell-Törmänen K, Bjermer L, Malmström A, Karlsson JC, Westergren-Thorsson G. Versican in inflammation and tissue remodeling: the impact on lung disorders. Glycobiology 25: 243–251, 2015. doi:10.1093/glycob/cwu120. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

