Flavonoid allosteric modulation of mutated visual rhodopsin associated with retinitis pigmentosa
- PMID: 28894166
- PMCID: PMC5593859
- DOI: 10.1038/s41598-017-11391-x
Flavonoid allosteric modulation of mutated visual rhodopsin associated with retinitis pigmentosa
Abstract
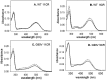
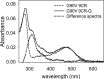
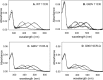
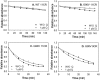
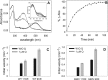
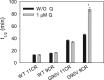
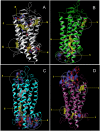
Dietary flavonoids exhibit many biologically-relevant functions and can potentially have beneficial effects in the treatment of pathological conditions. In spite of its well known antioxidant properties, scarce structural information is available on the interaction of flavonoids with membrane receptors. Advances in the structural biology of a specific class of membrane receptors, the G protein-coupled receptors, have significantly increased our understanding of drug action and paved the way for developing improved therapeutic approaches. We have analyzed the effect of the flavonoid quercetin on the conformation, stability and function of the G protein-coupled receptor rhodopsin, and the G90V mutant associated with the retinal degenerative disease retinitis pigmentosa. By using a combination of experimental and computational methods, we suggest that quercetin can act as an allosteric modulator of opsin regenerated with 9-cis-retinal and more importantly, that this binding has a positive effect on the stability and conformational properties of the G90V mutant associated with retinitis pigmentosa. These results open new possibilities to use quercetin and other flavonoids, in combination with specific retinoids like 9-cis-retinal, for the treatment of retinal degeneration associated with retinitis pigmentosa. Moreover, the use of flavonoids as allosteric modulators may also be applicable to other members of the G protein-coupled receptors superfamily.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Flavonoids improve the stability and function of P23H rhodopsin slowing down the progression of retinitis pigmentosa in mice.J Neurosci Res. 2022 Apr;100(4):1063-1083. doi: 10.1002/jnr.25021. Epub 2022 Feb 15. J Neurosci Res. 2022. PMID: 35165923 Free PMC article.
-
Docosahexaenoic acid phospholipid differentially modulates the conformation of G90V and N55K rhodopsin mutants associated with retinitis pigmentosa.Biochim Biophys Acta Biomembr. 2017 May;1859(5):975-981. doi: 10.1016/j.bbamem.2017.02.006. Epub 2017 Feb 16. Biochim Biophys Acta Biomembr. 2017. PMID: 28212859
-
Flavonoids enhance rod opsin stability, folding, and self-association by directly binding to ligand-free opsin and modulating its conformation.J Biol Chem. 2019 May 17;294(20):8101-8122. doi: 10.1074/jbc.RA119.007808. Epub 2019 Apr 3. J Biol Chem. 2019. PMID: 30944172 Free PMC article.
-
Rhodopsin as a Molecular Target to Mitigate Retinitis Pigmentosa.Adv Exp Med Biol. 2022;1371:61-77. doi: 10.1007/5584_2021_682. Adv Exp Med Biol. 2022. PMID: 34962636 Review.
-
Rhodopsin, Zn(2+), and retinitis pigmentosa: a short tale requiring continuation.Biochemistry (Mosc). 2013 Jun;78(6):660-6. doi: 10.1134/S0006297913060114. Biochemistry (Mosc). 2013. PMID: 23980892 Review.
Cited by
-
Luteolin delays photoreceptor degeneration in a mouse model of retinitis pigmentosa.Neural Regen Res. 2021 Oct;16(10):2109-2120. doi: 10.4103/1673-5374.303537. Neural Regen Res. 2021. PMID: 33642401 Free PMC article.
-
Flavonoids improve the stability and function of P23H rhodopsin slowing down the progression of retinitis pigmentosa in mice.J Neurosci Res. 2022 Apr;100(4):1063-1083. doi: 10.1002/jnr.25021. Epub 2022 Feb 15. J Neurosci Res. 2022. PMID: 35165923 Free PMC article.
-
New insights into the molecular mechanism of rhodopsin retinitis pigmentosa from the biochemical and functional characterization of G90V, Y102H and I307N mutations.Cell Mol Life Sci. 2022 Jan 7;79(1):58. doi: 10.1007/s00018-021-04086-0. Cell Mol Life Sci. 2022. PMID: 34997336 Free PMC article.
-
Retinoid analogs and polyphenols as potential therapeutics for age-related macular degeneration.Exp Biol Med (Maywood). 2020 Nov;245(17):1615-1625. doi: 10.1177/1535370220926938. Epub 2020 May 21. Exp Biol Med (Maywood). 2020. PMID: 32438835 Free PMC article. Review.
-
Polyphenols and Visual Health: Potential Effects on Degenerative Retinal Diseases.Molecules. 2021 Jun 4;26(11):3407. doi: 10.3390/molecules26113407. Molecules. 2021. PMID: 34199888 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

