PD1 signal transduction pathways in T cells
- PMID: 28881701
- PMCID: PMC5584302
- DOI: 10.18632/oncotarget.17232
PD1 signal transduction pathways in T cells
Abstract
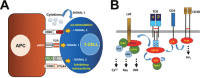
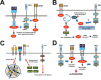
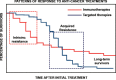
The use of immune checkpoint inhibitors for the treatment of cancer is revolutionizing oncology. Amongst these therapeutic agents, antibodies that block PD-L1/PD1 interactions between cancer cells and T cells are demonstrating high efficacies and low toxicities. Despite all the recent advances, very little is yet known on the molecular intracellular signaling pathways regulated by either PD-L1 or PD1. Here we review the current knowledge on PD1-dependent intracellular signaling pathways, and the consequences of disrupting PD1 signal transduction.
Keywords: B7-H1; PD-L1; PD1; PDL1; cancer.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures
Similar articles
-
Molecular mechanisms of programmed cell death-1 dependent T cell suppression: relevance for immunotherapy.Ann Transl Med. 2017 Oct;5(19):385. doi: 10.21037/atm.2017.06.11. Ann Transl Med. 2017. PMID: 29114543 Free PMC article. Review.
-
PDL1 Signals through Conserved Sequence Motifs to Overcome Interferon-Mediated Cytotoxicity.Cell Rep. 2017 Aug 22;20(8):1818-1829. doi: 10.1016/j.celrep.2017.07.075. Cell Rep. 2017. PMID: 28834746
-
Functional Expression of Programmed Death-Ligand 1 (B7-H1) by Immune Cells and Tumor Cells.Front Immunol. 2017 Aug 10;8:961. doi: 10.3389/fimmu.2017.00961. eCollection 2017. Front Immunol. 2017. PMID: 28848559 Free PMC article. Review.
-
A potential role for the PD1/PD-L1 pathway in the neuroinflammation of Alzheimer's disease.Neurobiol Aging. 2012 Mar;33(3):624.e11-22. doi: 10.1016/j.neurobiolaging.2011.03.004. Epub 2011 Apr 22. Neurobiol Aging. 2012. PMID: 21514692
-
Immunomodulating antibodies in the treatment of metastatic melanoma: the experience with anti-CTLA-4, anti-CD137, and anti-PD1.J Immunotoxicol. 2012 Jul-Sep;9(3):241-7. doi: 10.3109/1547691X.2012.678021. Epub 2012 Apr 23. J Immunotoxicol. 2012. PMID: 22524673 Review.
Cited by
-
Small molecule inhibitors against PD-1/PD-L1 immune checkpoints and current methodologies for their development: a review.Cancer Cell Int. 2021 Apr 27;21(1):239. doi: 10.1186/s12935-021-01946-4. Cancer Cell Int. 2021. PMID: 33906641 Free PMC article. Review.
-
A Novel Selective Axl/Mer/CSF1R Kinase Inhibitor as a Cancer Immunotherapeutic Agent Targeting Both Immune and Tumor Cells in the Tumor Microenvironment.Cancers (Basel). 2022 Oct 2;14(19):4821. doi: 10.3390/cancers14194821. Cancers (Basel). 2022. PMID: 36230744 Free PMC article.
-
Systemic CD4 Immunity and PD-L1/PD-1 Blockade Immunotherapy.Int J Mol Sci. 2022 Oct 31;23(21):13241. doi: 10.3390/ijms232113241. Int J Mol Sci. 2022. PMID: 36362027 Free PMC article. Review.
-
From Super-Enhancer Non-coding RNA to Immune Checkpoint: Frameworks to Functions.Front Oncol. 2019 Nov 22;9:1307. doi: 10.3389/fonc.2019.01307. eCollection 2019. Front Oncol. 2019. PMID: 31824865 Free PMC article. Review.
-
Resistance to PD-L1/PD-1 Blockade Immunotherapy. A Tumor-Intrinsic or Tumor-Extrinsic Phenomenon?Front Pharmacol. 2020 Apr 7;11:441. doi: 10.3389/fphar.2020.00441. eCollection 2020. Front Pharmacol. 2020. PMID: 32317979 Free PMC article. Review.
References
-
- Gato-Cañas M, Arasanz H, Blanco-Luquin I, Glaría E, Arteta-Sanchez V, Kochan G, Escors D. Novel immunotherapies for the treatment of melanoma. Immunotherapy. 2016;8:613–32. - PubMed
-
- Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–45. - PubMed
-
- Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003;171:5165–71. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

