Protective immunity differs between routes of administration of attenuated malaria parasites independent of parasite liver load
- PMID: 28871201
- PMCID: PMC5583236
- DOI: 10.1038/s41598-017-10480-1
Protective immunity differs between routes of administration of attenuated malaria parasites independent of parasite liver load
Abstract
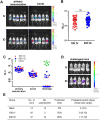
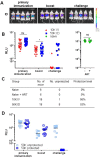
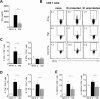
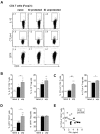
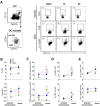
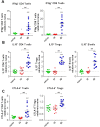
In humans and murine models of malaria, intradermal immunization (ID-I) with genetically attenuated sporozoites that arrest in liver induces lower protective immunity than intravenous immunization (IV-I). It is unclear whether this difference is caused by fewer sporozoites migrating into the liver or by suboptimal hepatic and injection site-dependent immune responses. We therefore developed a Plasmodium yoelii immunization/boost/challenge model to examine parasite liver loads as well as hepatic and lymph node immune responses in protected and unprotected ID-I and IV-I animals. Despite introducing the same numbers of genetically attenuated parasites in the liver, ID-I resulted in lower sterile protection (53-68%) than IV-I (93-95%). Unprotected mice developed less sporozoite-specific CD8+ and CD4+ effector T-cell responses than protected mice. After immunization, ID-I mice showed more interleukin-10-producing B and T cells in livers and skin-draining lymph nodes, but fewer hepatic CD8 memory T cells and CD8+ dendritic cells compared to IV-I mice. Our results indicate that the lower protection efficacy obtained by intradermal sporozoite administration is not linked to low hepatic parasite numbers as presumed before, but correlates with a shift towards regulatory immune responses. Overcoming these immune suppressive responses is important not only for live-attenuated malaria vaccines but also for other live vaccines administered in the skin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Parasite load stemming from immunization route determines the duration of liver-stage immunity.Parasite Immunol. 2019 Jul;41(7):e12622. doi: 10.1111/pim.12622. Epub 2019 Apr 4. Parasite Immunol. 2019. PMID: 30854655 Free PMC article.
-
Reduced Plasmodium berghei sporozoite liver load associates with low protective efficacy after intradermal immunization.Parasite Immunol. 2012 Dec;34(12):562-9. doi: 10.1111/pim.12000.x. Parasite Immunol. 2012. PMID: 23171040
-
Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells.J Infect Dis. 2007 Aug 15;196(4):608-16. doi: 10.1086/519742. Epub 2007 Jul 9. J Infect Dis. 2007. PMID: 17624848
-
Protective CD8 T cells against Plasmodium liver stages: immunobiology of an 'unnatural' immune response.Immunol Rev. 2008 Oct;225:272-83. doi: 10.1111/j.1600-065X.2008.00671.x. Immunol Rev. 2008. PMID: 18837788 Free PMC article. Review.
-
Immune response to pre-erythrocytic stages of malaria parasites.Curr Mol Med. 2006 Mar;6(2):169-85. doi: 10.2174/156652406776055249. Curr Mol Med. 2006. PMID: 16515509 Review.
Cited by
-
Plasmodium sporozoites induce regulatory macrophages.PLoS Pathog. 2020 Sep 8;16(9):e1008799. doi: 10.1371/journal.ppat.1008799. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32898164 Free PMC article.
-
Quantification of wild-type and radiation attenuated Plasmodium falciparum sporozoite motility in human skin.Sci Rep. 2019 Sep 17;9(1):13436. doi: 10.1038/s41598-019-49895-3. Sci Rep. 2019. PMID: 31530862 Free PMC article.
-
Malaria: influence of Anopheles mosquito saliva on Plasmodium infection.Trends Immunol. 2023 Apr;44(4):256-265. doi: 10.1016/j.it.2023.02.005. Epub 2023 Mar 22. Trends Immunol. 2023. PMID: 36964020 Free PMC article. Review.
-
A tracer-based method enables tracking of Plasmodium falciparum malaria parasites during human skin infection.Theranostics. 2019 Apr 13;9(10):2768-2778. doi: 10.7150/thno.33467. eCollection 2019. Theranostics. 2019. PMID: 31244921 Free PMC article.
-
Clustering and Erratic Movement Patterns of Syringe-Injected versus Mosquito-Inoculated Malaria Sporozoites Underlie Decreased Infectivity.mSphere. 2021 Apr 7;6(2):e00218-21. doi: 10.1128/mSphere.00218-21. mSphere. 2021. PMID: 33827910 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

