Deletion of c-FLIP from CD11bhi Macrophages Prevents Development of Bleomycin-induced Lung Fibrosis
- PMID: 28850249
- PMCID: PMC5941310
- DOI: 10.1165/rcmb.2017-0154OC
Deletion of c-FLIP from CD11bhi Macrophages Prevents Development of Bleomycin-induced Lung Fibrosis
Abstract
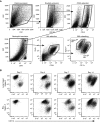
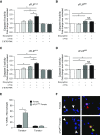
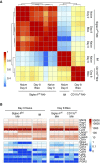
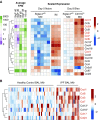
Idiopathic pulmonary fibrosis is a progressive lung disease with complex pathophysiology and fatal prognosis. Macrophages (MΦ) contribute to the development of lung fibrosis; however, the underlying mechanisms and specific MΦ subsets involved remain unclear. During lung injury, two subsets of lung MΦ coexist: Siglec-Fhi resident alveolar MΦ and a mixed population of CD11bhi MΦ that primarily mature from immigrating monocytes. Using a novel inducible transgenic system driven by a fragment of the human CD68 promoter, we targeted deletion of the antiapoptotic protein cellular FADD-like IL-1β-converting enzyme-inhibitory protein (c-FLIP) to CD11bhi MΦ. Upon loss of c-FLIP, CD11bhi MΦ became susceptible to cell death. Using this system, we were able to show that eliminating CD11bhi MΦ present 7-14 days after bleomycin injury was sufficient to protect mice from fibrosis. RNA-seq analysis of lung MΦ present during this time showed that CD11bhi MΦ, but not Siglec-Fhi MΦ, expressed high levels of profibrotic chemokines and growth factors. Human MΦ from patients with idiopathic pulmonary fibrosis expressed many of the same profibrotic chemokines identified in murine CD11bhi MΦ. Elimination of monocyte-derived MΦ may help in the treatment of fibrosis. We identify c-FLIP and the associated extrinsic cell death program as a potential pathway through which these profibrotic MΦ may be pharmacologically targeted.
Keywords: IPF; RNA-seq; c-FLIP; fibrosis; macrophage.
Figures




Similar articles
-
Myeloid cell dynamics in bleomycin-induced pulmonary injury in mice; effects of anti-TNFα antibody.Toxicol Appl Pharmacol. 2021 Apr 15;417:115470. doi: 10.1016/j.taap.2021.115470. Epub 2021 Feb 27. Toxicol Appl Pharmacol. 2021. PMID: 33647319 Free PMC article.
-
HIF1A up-regulates the ADORA2B receptor on alternatively activated macrophages and contributes to pulmonary fibrosis.FASEB J. 2017 Nov;31(11):4745-4758. doi: 10.1096/fj.201700219R. Epub 2017 Jul 12. FASEB J. 2017. PMID: 28701304 Free PMC article.
-
Paired immunoglobulin-like receptor-B inhibits pulmonary fibrosis by suppressing profibrogenic properties of alveolar macrophages.Am J Respir Cell Mol Biol. 2013 Apr;48(4):456-64. doi: 10.1165/rcmb.2012-0329OC. Am J Respir Cell Mol Biol. 2013. PMID: 23258232
-
Sphingolipids in pulmonary fibrosis.Adv Biol Regul. 2015 Jan;57:55-63. doi: 10.1016/j.jbior.2014.09.008. Epub 2014 Oct 13. Adv Biol Regul. 2015. PMID: 25446881 Free PMC article. Review.
-
A role for C-C chemokines in fibrotic lung disease.J Leukoc Biol. 1995 May;57(5):782-7. doi: 10.1002/jlb.57.5.782. J Leukoc Biol. 1995. PMID: 7539030 Review.
Cited by
-
Resetting proteostasis with ISRIB promotes epithelial differentiation to attenuate pulmonary fibrosis.Proc Natl Acad Sci U S A. 2021 May 18;118(20):e2101100118. doi: 10.1073/pnas.2101100118. Proc Natl Acad Sci U S A. 2021. PMID: 33972447 Free PMC article.
-
Origin and ontogeny of lung macrophages: from mice to humans.Immunology. 2020 Jun;160(2):126-138. doi: 10.1111/imm.13154. Epub 2019 Dec 4. Immunology. 2020. PMID: 31715003 Free PMC article. Review.
-
The arginine methyltransferase PRMT7 promotes extravasation of monocytes resulting in tissue injury in COPD.Nat Commun. 2022 Mar 14;13(1):1303. doi: 10.1038/s41467-022-28809-4. Nat Commun. 2022. PMID: 35288557 Free PMC article.
-
Noninvasive assessment of the lung inflammation-fibrosis axis by targeted imaging of CMKLR1.Sci Adv. 2024 Jun 21;10(25):eadm9817. doi: 10.1126/sciadv.adm9817. Epub 2024 Jun 19. Sci Adv. 2024. PMID: 38896611 Free PMC article.
-
CXCL11 reprograms M2-biased macrophage polarization to alleviate pulmonary fibrosis in mice.Cell Biosci. 2024 Nov 15;14(1):140. doi: 10.1186/s13578-024-01320-7. Cell Biosci. 2024. PMID: 39548525 Free PMC article.
References
-
- Schwartz DA, Helmers RA, Dayton CS, Merchant RK, Hunninghake GW. Determinants of bronchoalveolar lavage cellularity in idiopathic pulmonary fibrosis. 1991;71:1688–1693. - PubMed
-
- Byrne AJ, Maher TM, Lloyd CM. Pulmonary macrophages: a new therapeutic pathway in fibrosing lung disease? 2016;22:303–316. - PubMed
-
- Scott CL, Henri S, Guilliams M. Mononuclear phagocytes of the intestine, the skin, and the lung. 2014;262:9–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

