Advances in Immunotherapy for Melanoma: A Comprehensive Review
- PMID: 28848246
- PMCID: PMC5564072
- DOI: 10.1155/2017/3264217
Advances in Immunotherapy for Melanoma: A Comprehensive Review
Abstract
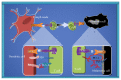
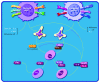
Melanomas are tumors originating from melanocytes and tend to show early metastasis secondary to the loss of cellular adhesion in the primary tumor, resulting in high mortality rates. Cancer-specific active immunotherapy is an experimental form of treatment that stimulates the immune system to recognize antigens on the surface of cancer cells. Current experimental approaches in immunotherapy include vaccines, biochemotherapy, and the transfer of adoptive T cells and dendritic cells. Several types of vaccines, including peptide, viral, and dendritic cell vaccines, are currently under investigation for the treatment of melanoma. These treatments have the same goal as drugs that are already used to stimulate the proliferation of T lymphocytes in order to destroy tumor cells; however, immunotherapies aim to selectively attack the tumor cells of each patient. In this comprehensive review, we describe recent advancements in the development of immunotherapies for melanoma, with a specific focus on the identification of neoantigens for the prediction of their elicited immune responses. This review is expected to provide important insights into the future of immunotherapy for melanoma.
Figures


Similar articles
-
Immune responses to a class II helper peptide epitope in patients with stage III/IV resected melanoma.Clin Cancer Res. 2004 Aug 1;10(15):5004-13. doi: 10.1158/1078-0432.CCR-04-0241. Clin Cancer Res. 2004. PMID: 15297401 Clinical Trial.
-
Randomized phase II trial of lymphodepletion plus adoptive cell transfer of tumor-infiltrating lymphocytes, with or without dendritic cell vaccination, in patients with metastatic melanoma.J Immunother Cancer. 2021 May;9(5):e002449. doi: 10.1136/jitc-2021-002449. J Immunother Cancer. 2021. PMID: 34021033 Free PMC article. Clinical Trial.
-
Strategies to overcome obstacles to successful immunotherapy of melanoma.Int J Immunopathol Pharmacol. 2008 Jul-Sep;21(3):493-500. doi: 10.1177/039463200802100302. Int J Immunopathol Pharmacol. 2008. PMID: 18831916 Free PMC article. Review.
-
Update on immunotherapy for melanoma.J Natl Compr Canc Netw. 2006 Aug;4(7):687-94. doi: 10.6004/jnccn.2006.0058. J Natl Compr Canc Netw. 2006. PMID: 16884670 Review.
-
A role of immunotherapy in metastatic malignant melanoma.Cent Nerv Syst Agents Med Chem. 2012 Sep;12(3):182-8. doi: 10.2174/187152412802430165. Cent Nerv Syst Agents Med Chem. 2012. PMID: 22697295 Review.
Cited by
-
Associations between dysbiosis-inducing drugs, overall survival and tumor response in patients treated with immune checkpoint inhibitors.Ther Adv Med Oncol. 2021 Mar 15;13:17588359211000591. doi: 10.1177/17588359211000591. eCollection 2021. Ther Adv Med Oncol. 2021. PMID: 33796151 Free PMC article.
-
Enhancing Skin Cancer Immunotheranostics and Precision Medicine through Functionalized Nanomodulators and Nanosensors: Recent Development and Prospects.Int J Mol Sci. 2023 Feb 9;24(4):3493. doi: 10.3390/ijms24043493. Int J Mol Sci. 2023. PMID: 36834917 Free PMC article. Review.
-
First-line Advanced Cutaneous Melanoma Treatments: Where Do We Stand?JMIR Cancer. 2021 Dec 15;7(4):e29912. doi: 10.2196/29912. JMIR Cancer. 2021. PMID: 34914610 Free PMC article.
-
Recent Progress in Nanomedicine for Melanoma Theranostics With Emphasis on Combination Therapy.Front Bioeng Biotechnol. 2021 Mar 11;9:661214. doi: 10.3389/fbioe.2021.661214. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33777924 Free PMC article. Review.
-
Novel therapeutic strategy for melanoma based on albendazole and the CDK4/6 inhibitor palbociclib.Sci Rep. 2022 Apr 5;12(1):5706. doi: 10.1038/s41598-022-09592-0. Sci Rep. 2022. PMID: 35383224 Free PMC article.
References
-
- Kaufman K. L., Mactier S., Armstrong N. J., et al. Surface antigen profiles of leukocytes and melanoma cells in lymph node metastases are associated with survival in AJCC stage III melanoma patients. Clinical & Experimental Metastasis. 2014;31(4):407–421. doi: 10.1007/s10585-014-9636-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

