A20 Restrains Thymic Regulatory T Cell Development
- PMID: 28842469
- PMCID: PMC5617121
- DOI: 10.4049/jimmunol.1602102
A20 Restrains Thymic Regulatory T Cell Development
Abstract
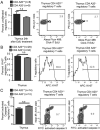
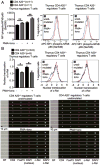
Maintaining immune tolerance requires the production of Foxp3-expressing regulatory T (Treg) cells in the thymus. Activation of NF-κB transcription factors is critically required for Treg cell development, partly via initiating Foxp3 expression. NF-κB activation is controlled by a negative feedback regulation through the ubiquitin editing enzyme A20, which reduces proinflammatory signaling in myeloid cells and B cells. In naive CD4+ T cells, A20 prevents kinase RIPK3-dependent necroptosis. Using mice deficient for A20 in T lineage cells, we show that thymic and peripheral Treg cell compartments are quantitatively enlarged because of a cell-intrinsic developmental advantage of A20-deficient thymic Treg differentiation. A20-deficient thymic Treg cells exhibit reduced dependence on IL-2 but unchanged rates of proliferation and apoptosis. Activation of the NF-κB transcription factor RelA was enhanced, whereas nuclear translocation of c-Rel was decreased in A20-deficient thymic Treg cells. Furthermore, we found that the increase in Treg cells in T cell-specific A20-deficient mice was already observed in CD4+ single-positive CD25+ GITR+ Foxp3- thymic Treg cell progenitors. Treg cell precursors expressed high levels of the tumor necrosis factor receptor superfamily molecule GITR, whose stimulation is closely linked to thymic Treg cell development. A20-deficient Treg cells efficiently suppressed effector T cell-mediated graft-versus-host disease after allogeneic hematopoietic stem cell transplantation, suggesting normal suppressive function. Holding thymic production of natural Treg cells in check, A20 thus integrates Treg cell activity and increased effector T cell survival into an efficient CD4+ T cell response.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures

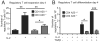

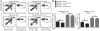

Similar articles
-
c-REL and IκBNS Govern Common and Independent Steps of Regulatory T Cell Development from Novel CD122-Expressing Pre-Precursors.J Immunol. 2017 Aug 1;199(3):920-930. doi: 10.4049/jimmunol.1600877. Epub 2017 Jun 26. J Immunol. 2017. PMID: 28652399
-
Generation of Foxp3+CD25- Regulatory T-Cell Precursors Requires c-Rel and IκBNS.Front Immunol. 2019 Jul 10;10:1583. doi: 10.3389/fimmu.2019.01583. eCollection 2019. Front Immunol. 2019. PMID: 31354726 Free PMC article.
-
Glucocorticoid hormone differentially modulates the in vitro expansion and cytokine profile of thymic and splenic Treg cells.Immunobiology. 2019 Mar;224(2):285-295. doi: 10.1016/j.imbio.2018.12.002. Epub 2018 Dec 27. Immunobiology. 2019. PMID: 30612787
-
c-Rel: a pioneer in directing regulatory T-cell lineage commitment?Eur J Immunol. 2010 Mar;40(3):664-7. doi: 10.1002/eji.201040372. Eur J Immunol. 2010. PMID: 20162555 Review.
-
Thymic commitment of regulatory T cells is a pathway of TCR-dependent selection that isolates repertoires undergoing positive or negative selection.Curr Top Microbiol Immunol. 2005;293:43-71. doi: 10.1007/3-540-27702-1_3. Curr Top Microbiol Immunol. 2005. PMID: 15981475 Review.
Cited by
-
Tumour necrosis factor signalling in health and disease.F1000Res. 2019 Jan 28;8:F1000 Faculty Rev-111. doi: 10.12688/f1000research.17023.1. eCollection 2019. F1000Res. 2019. PMID: 30755793 Free PMC article. Review.
-
Deubiquitinating Enzyme: A Potential Secondary Checkpoint of Cancer Immunity.Front Oncol. 2020 Aug 7;10:1289. doi: 10.3389/fonc.2020.01289. eCollection 2020. Front Oncol. 2020. PMID: 32850399 Free PMC article. Review.
-
Notch and NF-κB: Coach and Players of Regulatory T-Cell Response in Cancer.Front Immunol. 2018 Oct 11;9:2165. doi: 10.3389/fimmu.2018.02165. eCollection 2018. Front Immunol. 2018. PMID: 30364244 Free PMC article. Review.
-
A20: A multifunctional tool for regulating immunity and preventing disease.Cell Immunol. 2019 Jun;340:103914. doi: 10.1016/j.cellimm.2019.04.002. Epub 2019 Apr 5. Cell Immunol. 2019. PMID: 31030956 Free PMC article. Review.
-
Ubiquitin-Dependent Regulation of Treg Function and Plasticity.Adv Exp Med Biol. 2021;1278:63-80. doi: 10.1007/978-981-15-6407-9_4. Adv Exp Med Biol. 2021. PMID: 33523443
References
-
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. - PubMed
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
-
- Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12:157–167. - PubMed
-
- Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol. 2009;9:83–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

