Indoles from commensal bacteria extend healthspan
- PMID: 28827345
- PMCID: PMC5594673
- DOI: 10.1073/pnas.1706464114
Indoles from commensal bacteria extend healthspan
Abstract
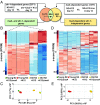
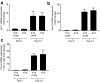
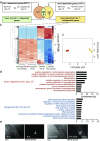
Multiple studies have identified conserved genetic pathways and small molecules associated with extension of lifespan in diverse organisms. However, extending lifespan does not result in concomitant extension in healthspan, defined as the proportion of time that an animal remains healthy and free of age-related infirmities. Rather, mutations that extend lifespan often reduce healthspan and increase frailty. The question arises as to whether factors or mechanisms exist that uncouple these processes and extend healthspan and reduce frailty independent of lifespan. We show that indoles from commensal microbiota extend healthspan of diverse organisms, including Caenorhabditis elegans, Drosophila melanogaster, and mice, but have a negligible effect on maximal lifespan. Effects of indoles on healthspan in worms and flies depend upon the aryl hydrocarbon receptor (AHR), a conserved detector of xenobiotic small molecules. In C. elegans, indole induces a gene expression profile in aged animals reminiscent of that seen in the young, but which is distinct from that associated with normal aging. Moreover, in older animals, indole induces genes associated with oogenesis and, accordingly, extends fecundity and reproductive span. Together, these data suggest that small molecules related to indole and derived from commensal microbiota act in diverse phyla via conserved molecular pathways to promote healthy aging. These data raise the possibility of developing therapeutics based on microbiota-derived indole or its derivatives to extend healthspan and reduce frailty in humans.
Keywords: C. elegans; aging; aryl hydrocarbon receptor; frailty; microbiota.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





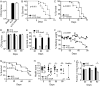
Similar articles
-
Involvement of Aryl Hydrocarbon Receptor in Longevity and Healthspan: Insights from Humans, Mice, and C. elegans.Int J Mol Sci. 2024 Sep 14;25(18):9943. doi: 10.3390/ijms25189943. Int J Mol Sci. 2024. PMID: 39337431 Free PMC article.
-
Dietary and environmental factors have opposite AhR-dependent effects on C. elegans healthspan.Aging (Albany NY). 2020 Dec 13;13(1):104-133. doi: 10.18632/aging.202316. Epub 2020 Dec 13. Aging (Albany NY). 2020. PMID: 33349622 Free PMC article.
-
Movement decline across lifespan of Caenorhabditis elegans mutants in the insulin/insulin-like signaling pathway.Aging Cell. 2018 Feb;17(1):e12704. doi: 10.1111/acel.12704. Epub 2017 Dec 7. Aging Cell. 2018. PMID: 29214707 Free PMC article.
-
The role of lipid metabolism in aging, lifespan regulation, and age-related disease.Aging Cell. 2019 Dec;18(6):e13048. doi: 10.1111/acel.13048. Epub 2019 Sep 27. Aging Cell. 2019. PMID: 31560163 Free PMC article. Review.
-
Healthspan pathway maps in C. elegans and humans highlight transcription, proliferation/biosynthesis and lipids.Aging (Albany NY). 2020 Jul 7;12(13):12534-12581. doi: 10.18632/aging.103514. Epub 2020 Jul 7. Aging (Albany NY). 2020. PMID: 32634117 Free PMC article. Review.
Cited by
-
Microbial dynamics, chemical profile, and bioactive potential of diverse Egyptian marine environments from archaeological wood to soda lake.Sci Rep. 2024 Sep 9;14(1):20918. doi: 10.1038/s41598-024-70411-9. Sci Rep. 2024. PMID: 39251732 Free PMC article.
-
Structural Snapshots of Proteus vulgaris Tryptophan Indole-Lyase Reveal Insights into the Catalytic Mechanism.ACS Catal. 2024 Jul 18;14(15):11498-11511. doi: 10.1021/acscatal.4c03232. eCollection 2024 Aug 2. ACS Catal. 2024. PMID: 39114092 Free PMC article.
-
Gut microbiota-astrocyte axis: new insights into age-related cognitive decline.Neural Regen Res. 2025 Apr 1;20(4):990-1008. doi: 10.4103/NRR.NRR-D-23-01776. Epub 2024 Apr 16. Neural Regen Res. 2025. PMID: 38989933 Free PMC article.
-
Microbial Indoles: Key Regulators of Organ Growth and Metabolic Function.Microorganisms. 2024 Apr 2;12(4):719. doi: 10.3390/microorganisms12040719. Microorganisms. 2024. PMID: 38674663 Free PMC article.
-
Changes in Bacterial Gut Composition in Parkinson's Disease and Their Metabolic Contribution to Disease Development: A Gut Community Reconstruction Approach.Microorganisms. 2024 Feb 4;12(2):325. doi: 10.3390/microorganisms12020325. Microorganisms. 2024. PMID: 38399728 Free PMC article.
References
-
- Meara E, White C, Cutler DM. Trends in medical spending by age, 1963–2000. Health Aff (Millwood) 2004;23:176–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

