MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis
- PMID: 28827318
- PMCID: PMC5594682
- DOI: 10.1073/pnas.1707531114
MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis
Abstract
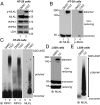
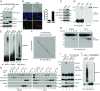
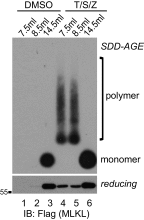
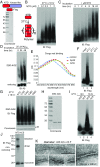
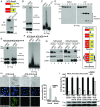
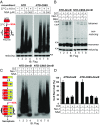
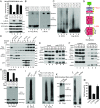
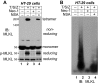
Mixed-lineage kinase domain-like protein (MLKL) is essential for TNF-α-induced necroptosis. How MLKL promotes cell death is still under debate. Here we report that MLKL forms SDS-resistant, disulfide bond-dependent polymers during necroptosis in both human and mouse cells. MLKL polymers are independent of receptor-interacting protein kinase 1 and 3 (RIPK1/RIPK3) fibers. Large MLKL polymers are more than 2 million Da and are resistant to proteinase K digestion. MLKL polymers are fibers 5 nm in diameter under electron microscopy. Furthermore, the recombinant N-terminal domain of MLKL forms amyloid-like fibers and binds Congo red dye. MLKL mutants that cannot form polymers also fail to induce necroptosis efficiently. Finally, the compound necrosulfonamide conjugates cysteine 86 of human MLKL and blocks MLKL polymer formation and subsequent cell death. These results demonstrate that disulfide bond-dependent, amyloid-like MLKL polymers are necessary and sufficient to induce necroptosis.
Keywords: MLKL; amyloid-like; disulfide bond; necroptosis; polymer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Thioredoxin-1 actively maintains the pseudokinase MLKL in a reduced state to suppress disulfide bond-dependent MLKL polymer formation and necroptosis.J Biol Chem. 2017 Oct 20;292(42):17514-17524. doi: 10.1074/jbc.M117.799353. Epub 2017 Sep 6. J Biol Chem. 2017. PMID: 28878015 Free PMC article.
-
CAMK2/CaMKII activates MLKL in short-term starvation to facilitate autophagic flux.Autophagy. 2022 Apr;18(4):726-744. doi: 10.1080/15548627.2021.1954348. Epub 2021 Jul 20. Autophagy. 2022. PMID: 34282994 Free PMC article.
-
Discovery of potent necroptosis inhibitors targeting RIPK1 kinase activity for the treatment of inflammatory disorder and cancer metastasis.Cell Death Dis. 2019 Jun 24;10(7):493. doi: 10.1038/s41419-019-1735-6. Cell Death Dis. 2019. PMID: 31235688 Free PMC article.
-
Viral-induced neuronal necroptosis: Detrimental to brain function and regulation by necroptosis inhibitors.Biochem Pharmacol. 2023 Jul;213:115591. doi: 10.1016/j.bcp.2023.115591. Epub 2023 May 16. Biochem Pharmacol. 2023. PMID: 37196683 Review.
-
Novel drug discovery strategies for atherosclerosis that target necrosis and necroptosis.Expert Opin Drug Discov. 2018 Jun;13(6):477-488. doi: 10.1080/17460441.2018.1457644. Epub 2018 Mar 29. Expert Opin Drug Discov. 2018. PMID: 29598451 Review.
Cited by
-
Pyroptosis in health and disease: mechanisms, regulation and clinical perspective.Signal Transduct Target Ther. 2024 Sep 20;9(1):245. doi: 10.1038/s41392-024-01958-2. Signal Transduct Target Ther. 2024. PMID: 39300122 Free PMC article. Review.
-
Role of necroptosis in traumatic brain and spinal cord injuries.J Adv Res. 2022 Sep;40:125-134. doi: 10.1016/j.jare.2021.12.002. Epub 2021 Dec 22. J Adv Res. 2022. PMID: 36100321 Free PMC article. Review.
-
Use of Two Dimensional Semi-denaturing Detergent Agarose Gel Electrophoresis to Confirm Size Heterogeneity of Amyloid or Amyloid-like Fibers.J Vis Exp. 2018 Apr 26;(134):57498. doi: 10.3791/57498. J Vis Exp. 2018. PMID: 29757289 Free PMC article.
-
Necroptosis in development and diseases.Genes Dev. 2018 Mar 1;32(5-6):327-340. doi: 10.1101/gad.312561.118. Genes Dev. 2018. PMID: 29593066 Free PMC article. Review.
-
Pyroptosis versus necroptosis: similarities, differences, and crosstalk.Cell Death Differ. 2019 Jan;26(1):99-114. doi: 10.1038/s41418-018-0212-6. Epub 2018 Oct 19. Cell Death Differ. 2019. PMID: 30341423 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

