Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion
- PMID: 28816238
- PMCID: PMC5843902
- DOI: 10.1038/leu.2017.259
Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion
Abstract
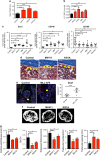
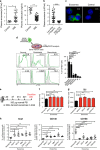
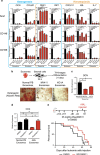
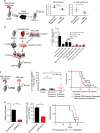
Little is known about how leukemia cells alter the bone marrow (BM) niche to facilitate their own growth and evade chemotherapy. Here, we provide evidence that acute myeloid leukemia (AML) blasts remodel the BM niche into a leukemia growth-permissive and normal hematopoiesis-suppressive microenvironment through exosome secretion. Either engrafted AML cells or AML-derived exosomes increased mesenchymal stromal progenitors and blocked osteolineage development and bone formation in vivo. Preconditioning with AML-derived exosomes 'primed' the animals for accelerated AML growth. Conversely, disruption of exosome secretion in AML cells through targeting Rab27a, an important regulator involved in exosome release, significantly delayed leukemia development. In BM stromal cells, AML-derived exosomes induced the expression of DKK1, a suppressor of normal hematopoiesis and osteogenesis, thereby contributing to osteoblast loss. Conversely, treatment with a DKK1 inhibitor delayed AML progression and prolonged survival in AML-engrafted mice. In addition, AML-derived exosomes induced a broad downregulation of hematopoietic stem cell-supporting factors (for example, CXCL12, KITL and IGF1) in BM stromal cells and reduced their ability to support normal hematopoiesis. Altogether, this study uncovers novel features of AML pathogenesis and unveils how AML cells create a self-strengthening leukemic niche that promotes leukemic cell proliferation and survival, while suppressing normal hematopoiesis through exosome secretion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Exosomes in acute myeloid leukemia inhibit hematopoiesis.Curr Opin Hematol. 2018 Jul;25(4):279-284. doi: 10.1097/MOH.0000000000000439. Curr Opin Hematol. 2018. PMID: 29846239 Review.
-
Alteration of cellular and immune-related properties of bone marrow mesenchymal stem cells and macrophages by K562 chronic myeloid leukemia cell derived exosomes.J Cell Physiol. 2019 Apr;234(4):3697-3710. doi: 10.1002/jcp.27142. Epub 2018 Oct 14. J Cell Physiol. 2019. PMID: 30317554
-
Mesenchymal stromal cells from patients with acute myeloid leukemia have altered capacity to expand differentiated hematopoietic progenitors.Leuk Res. 2015 Apr;39(4):486-93. doi: 10.1016/j.leukres.2015.01.013. Epub 2015 Feb 2. Leuk Res. 2015. PMID: 25703353
-
Distinct roles of mesenchymal stem and progenitor cells during the development of acute myeloid leukemia in mice.Blood Adv. 2018 Jun 26;2(12):1480-1494. doi: 10.1182/bloodadvances.2017013870. Blood Adv. 2018. PMID: 29945938 Free PMC article.
-
Roles of the bone marrow niche in hematopoiesis, leukemogenesis, and chemotherapy resistance in acute myeloid leukemia.Hematology. 2018 Dec;23(10):729-739. doi: 10.1080/10245332.2018.1486064. Epub 2018 Jun 14. Hematology. 2018. PMID: 29902132 Review.
Cited by
-
Moving Myeloid Leukemia Drug Discovery Into the Third Dimension.Front Pediatr. 2019 Jul 30;7:314. doi: 10.3389/fped.2019.00314. eCollection 2019. Front Pediatr. 2019. PMID: 31417884 Free PMC article. Review.
-
Role of tumor-derived exosomes in bone metastasis.Oncol Lett. 2019 Oct;18(4):3935-3945. doi: 10.3892/ol.2019.10776. Epub 2019 Aug 22. Oncol Lett. 2019. PMID: 31579412 Free PMC article. Review.
-
Current and Emerging Techniques for Diagnosis and MRD Detection in AML: A Comprehensive Narrative Review.Cancers (Basel). 2023 Feb 21;15(5):1362. doi: 10.3390/cancers15051362. Cancers (Basel). 2023. PMID: 36900154 Free PMC article. Review.
-
Quantification and Phenotypic Characterization of Extracellular Vesicles from Patients with Acute Myeloid and B-Cell Lymphoblastic Leukemia.Cancers (Basel). 2021 Dec 23;14(1):56. doi: 10.3390/cancers14010056. Cancers (Basel). 2021. PMID: 35008226 Free PMC article.
-
[Role of exosomes in leukemogenesis].Zhonghua Xue Ye Xue Za Zhi. 2019 Feb 14;40(2):173-176. doi: 10.3760/cma.j.issn.0253-2727.2019.02.017. Zhonghua Xue Ye Xue Za Zhi. 2019. PMID: 30831638 Free PMC article. Chinese. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

