Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets
- PMID: 28814629
- PMCID: PMC5584405
- DOI: 10.1073/pnas.1619216114
Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets
Abstract
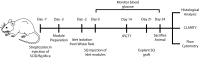
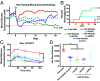
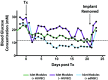
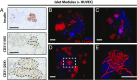
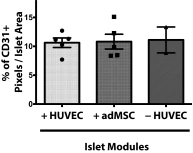
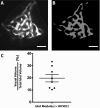
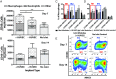
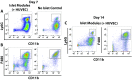
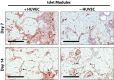
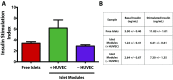
The transplantation of pancreatic islets, following the Edmonton Protocol, is a promising treatment for type I diabetics. However, the need for multiple donors to achieve insulin independence reflects the large loss of islets that occurs when islets are infused into the portal vein. Finding a less hostile transplantation site that is both minimally invasive and able to support a large transplant volume is necessary to advance this approach. Although the s.c. site satisfies both these criteria, the site is poorly vascularized, precluding its utility. To address this problem, we demonstrate that modular tissue engineering results in an s.c. vascularized bed that enables the transplantation of pancreatic islets. In streptozotocin-induced diabetic SCID/beige mice, the injection of 750 rat islet equivalents embedded in endothelialized collagen modules was sufficient to restore and maintain normoglycemia for 21 days; the same number of free islets was unable to affect glucose levels. Furthermore, using CLARITY, we showed that embedded islets became revascularized and integrated with the host's vasculature, a feature not seen in other s.c.
Studies: Collagen-embedded islets drove a small (albeit not significant) shift toward a proangiogenic CD206+MHCII-(M2-like) macrophage response, which was a feature of module-associated vascularization. While these results open the potential for using s.c. islet delivery as a treatment option for type I diabetes, the more immediate benefit may be for the exploration of revascularized islet biology.
Keywords: CLARITY; islet revascularization; pancreatic islets; subcutaneous transplant; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
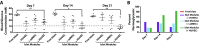
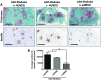
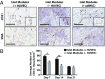
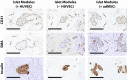
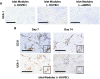
Similar articles
-
Endothelialized collagen based pseudo-islets enables tuneable subcutaneous diabetes therapy.Biomaterials. 2020 Feb;232:119710. doi: 10.1016/j.biomaterials.2019.119710. Epub 2019 Dec 26. Biomaterials. 2020. PMID: 31901691
-
Long-term insulin independence following repeated islet transplantation in totally pancreatectomized diabetic pigs.Cell Transplant. 2002;11(1):55-66. Cell Transplant. 2002. PMID: 12095221
-
A prevascularized tissue engineering chamber supports growth and function of islets and progenitor cells in diabetic mice.Islets. 2011 Sep-Oct;3(5):271-83. doi: 10.4161/isl.3.5.15942. Epub 2011 Sep 1. Islets. 2011. PMID: 21847009
-
Islet transplantation for Type 1 diabetes: where are we now?Expert Rev Clin Immunol. 2015 Jan;11(1):59-68. doi: 10.1586/1744666X.2015.978291. Epub 2014 Dec 2. Expert Rev Clin Immunol. 2015. PMID: 25454816 Review.
-
Hepatocyte growth factor gene therapy for islet transplantation.Expert Opin Biol Ther. 2004 Apr;4(4):507-18. doi: 10.1517/14712598.4.4.507. Expert Opin Biol Ther. 2004. PMID: 15102600 Review.
Cited by
-
Subcutaneous device-free islet transplantation.Front Immunol. 2023 Oct 18;14:1287182. doi: 10.3389/fimmu.2023.1287182. eCollection 2023. Front Immunol. 2023. PMID: 37965322 Free PMC article. Review.
-
Study on the Effect of PDA-PLGA Scaffold Loaded With Islet Cells for Skeletal Muscle Transplantation in the Treatment of Diabetes.Front Bioeng Biotechnol. 2022 Jun 30;10:927348. doi: 10.3389/fbioe.2022.927348. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35845408 Free PMC article.
-
Transplantation of Macroencapsulated Insulin-Producing Cells.Curr Diab Rep. 2018 Jun 16;18(8):50. doi: 10.1007/s11892-018-1028-y. Curr Diab Rep. 2018. PMID: 29909496 Review.
-
Noninvasive Tracking of mPEG-poly(Ala) Hydrogel-Embedded MIN6 Cells after Subcutaneous Transplantation in Mice.Polymers (Basel). 2021 Mar 13;13(6):885. doi: 10.3390/polym13060885. Polymers (Basel). 2021. PMID: 33805723 Free PMC article.
-
A novel bioartificial pancreas fabricated via islets microencapsulation in anti-adhesive core-shell microgels and macroencapsulation in a hydrogel scaffold prevascularized in vivo.Bioact Mater. 2023 Apr 20;27:362-376. doi: 10.1016/j.bioactmat.2023.04.011. eCollection 2023 Sep. Bioact Mater. 2023. PMID: 37180642 Free PMC article.
References
-
- Shapiro AMJ, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. - PubMed
-
- Berney T, et al. Long-term insulin-independence after allogeneic islet transplantation for type 1 diabetes: Over the 10-year mark. Am J Transplant. 2009;9:419–423. - PubMed
-
- Biarnés M, et al. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51:66–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

