Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade
- PMID: 28810147
- PMCID: PMC5568814
- DOI: 10.1016/j.ccell.2017.07.006
Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade
Abstract
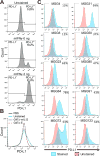
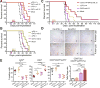
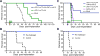
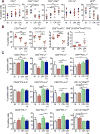
Glioblastoma is an immunosuppressive, fatal brain cancer that contains glioblastoma stem-like cells (GSCs). Oncolytic herpes simplex virus (oHSV) selectively replicates in cancer cells while inducing anti-tumor immunity. oHSV G47Δ expressing murine IL-12 (G47Δ-mIL12), antibodies to immune checkpoints (CTLA-4, PD-1, PD-L1), or dual combinations modestly extended survival of a mouse glioma model. However, the triple combination of anti-CTLA-4, anti-PD-1, and G47Δ-mIL12 cured most mice in two glioma models. This treatment was associated with macrophage influx and M1-like polarization, along with increased T effector to T regulatory cell ratios. Immune cell depletion studies demonstrated that CD4+ and CD8+ T cells as well as macrophages are required for synergistic curative activity. This combination should be translatable to the clinic and other immunosuppressive cancers.
Keywords: HSV; cancer stem cells; glioma; immunotherapy; oncolytic virus.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


Comment in
-
A Viro-Immunotherapy Triple Play for the Treatment of Glioblastoma.Cancer Cell. 2017 Aug 14;32(2):133-134. doi: 10.1016/j.ccell.2017.07.012. Cancer Cell. 2017. PMID: 28810141
Similar articles
-
Oncolytic herpes simplex virus immunovirotherapy in combination with immune checkpoint blockade to treat glioblastoma.Immunotherapy. 2018 Jul;10(9):779-786. doi: 10.2217/imt-2018-0009. Immunotherapy. 2018. PMID: 30008259 Free PMC article.
-
Immunovirotherapy with measles virus strains in combination with anti-PD-1 antibody blockade enhances antitumor activity in glioblastoma treatment.Neuro Oncol. 2017 Apr 1;19(4):493-502. doi: 10.1093/neuonc/now179. Neuro Oncol. 2017. PMID: 27663389 Free PMC article.
-
Combinatorial Effects of VEGFR Kinase Inhibitor Axitinib and Oncolytic Virotherapy in Mouse and Human Glioblastoma Stem-Like Cell Models.Clin Cancer Res. 2018 Jul 15;24(14):3409-3422. doi: 10.1158/1078-0432.CCR-17-1717. Epub 2018 Mar 29. Clin Cancer Res. 2018. PMID: 29599413 Free PMC article.
-
Abscopal effects observed in cancer radiation therapy and oncolytic virotherapy: an overview.Drugs Today (Barc). 2019 Feb;55(2):117-130. doi: 10.1358/dot.2019.55.2.2903217. Drugs Today (Barc). 2019. PMID: 30816886 Review.
-
Clinical Trials Investigating Immune Checkpoint Blockade in Glioblastoma.Curr Treat Options Oncol. 2017 Aug;18(8):51. doi: 10.1007/s11864-017-0492-y. Curr Treat Options Oncol. 2017. PMID: 28785997 Review.
Cited by
-
Interactions between cancer cells and immune cells drive transitions to mesenchymal-like states in glioblastoma.Cancer Cell. 2021 Jun 14;39(6):779-792.e11. doi: 10.1016/j.ccell.2021.05.002. Epub 2021 Jun 3. Cancer Cell. 2021. PMID: 34087162 Free PMC article.
-
Intratumoral expression of interleukin 23 variants using oncolytic vaccinia virus elicit potent antitumor effects on multiple tumor models via tumor microenvironment modulation.Theranostics. 2021 May 3;11(14):6668-6681. doi: 10.7150/thno.56494. eCollection 2021. Theranostics. 2021. PMID: 34093846 Free PMC article.
-
Neoadjuvant Use of Oncolytic Herpes Virus G47Δ Enhances the Antitumor Efficacy of Radiofrequency Ablation.Mol Ther Oncolytics. 2020 Aug 21;18:535-545. doi: 10.1016/j.omto.2020.08.010. eCollection 2020 Sep 25. Mol Ther Oncolytics. 2020. PMID: 32995479 Free PMC article.
-
Improved antitumor effectiveness of oncolytic HSV-1 viruses engineered with IL-15/IL-15Rα complex combined with oncolytic HSV-1-aPD1 targets colon cancer.Sci Rep. 2024 Oct 10;14(1):23671. doi: 10.1038/s41598-024-72888-w. Sci Rep. 2024. PMID: 39389985 Free PMC article.
-
Single-cell characterization of macrophages in glioblastoma reveals MARCO as a mesenchymal pro-tumor marker.Genome Med. 2021 May 19;13(1):88. doi: 10.1186/s13073-021-00906-x. Genome Med. 2021. PMID: 34011400 Free PMC article.
References
-
- Antonia SJ, Lopez-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jager D, Pietanza MC, Le DT, de Braud F, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016 - PubMed
-
- Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000;46:957–961. discussion 961–952. - PubMed
-
- Butowski N, Colman H, De Groot JF, Omuro AM, Nayak L, Wen PY, Cloughesy TF, Marimuthu A, Haidar S, Perry A, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol. 2016;18:557–564. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

