Influenza Virus Infection, Interferon Response, Viral Counter-Response, and Apoptosis
- PMID: 28805681
- PMCID: PMC5580480
- DOI: 10.3390/v9080223
Influenza Virus Infection, Interferon Response, Viral Counter-Response, and Apoptosis
Abstract
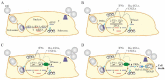
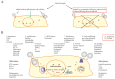
Human influenza A viruses (IAVs) cause global pandemics and epidemics, which remain serious threats to public health because of the shortage of effective means of control. To combat the surge of viral outbreaks, new treatments are urgently needed. Developing new virus control modalities requires better understanding of virus-host interactions. Here, we describe how IAV infection triggers cellular apoptosis and how this process can be exploited towards the development of new therapeutics, which might be more effective than the currently available anti-influenza drugs.
Keywords: antiviral agent; apoptosis; host response; influenza virus; innate immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Influenza A virus-induced apoptosis and virus propagation.Apoptosis. 2020 Feb;25(1-2):1-11. doi: 10.1007/s10495-019-01575-3. Apoptosis. 2020. PMID: 31667646 Review.
-
Efficient Inhibition of Avian and Seasonal Influenza A Viruses by a Virus-Specific Dicer-Substrate Small Interfering RNA Swarm in Human Monocyte-Derived Macrophages and Dendritic Cells.J Virol. 2019 Feb 5;93(4):e01916-18. doi: 10.1128/JVI.01916-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463970 Free PMC article.
-
Web of interferon stimulated antiviral factors to control the influenza A viruses replication.Microb Pathog. 2020 Feb;139:103919. doi: 10.1016/j.micpath.2019.103919. Epub 2019 Dec 10. Microb Pathog. 2020. PMID: 31830579 Review.
-
Innate Immune Response to Influenza Virus at Single-Cell Resolution in Human Epithelial Cells Revealed Paracrine Induction of Interferon Lambda 1.J Virol. 2019 Sep 30;93(20):e00559-19. doi: 10.1128/JVI.00559-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31375585 Free PMC article.
-
The Nonstructural NS1 Protein of Influenza Viruses Modulates TP53 Splicing through Host Factor CPSF4.J Virol. 2019 Mar 21;93(7):e02168-18. doi: 10.1128/JVI.02168-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651364 Free PMC article.
Cited by
-
Adult Immunization - Need of the Hour.J Int Soc Prev Community Dent. 2018 Nov-Dec;8(6):475-481. doi: 10.4103/jispcd.JISPCD_347_18. Epub 2018 Nov 29. J Int Soc Prev Community Dent. 2018. PMID: 30596036 Free PMC article. Review.
-
H3N2 influenza viruses in humans: Viral mechanisms, evolution, and evaluation.Hum Vaccin Immunother. 2018;14(8):1840-1847. doi: 10.1080/21645515.2018.1462639. Epub 2018 May 14. Hum Vaccin Immunother. 2018. PMID: 29641358 Free PMC article. Review.
-
Broadly Protective Neuraminidase-Based Influenza Vaccines and Monoclonal Antibodies: Target Epitopes and Mechanisms of Action.Viruses. 2023 Jan 10;15(1):200. doi: 10.3390/v15010200. Viruses. 2023. PMID: 36680239 Free PMC article. Review.
-
A Systems Approach to Study Immuno- and Neuro-Modulatory Properties of Antiviral Agents.Viruses. 2018 Aug 12;10(8):423. doi: 10.3390/v10080423. Viruses. 2018. PMID: 30103549 Free PMC article. Review.
-
Replication of Influenza A Virus in Secondary Lymphatic Tissue Contributes to Innate Immune Activation.Pathogens. 2021 May 19;10(5):622. doi: 10.3390/pathogens10050622. Pathogens. 2021. PMID: 34069514 Free PMC article.
References
-
- WHO Influenza (Seasonal) [(accessed on 8 July 2017)]; Available online: http://wwwwhoint/mediacentre/factsheets/fs211/en/
-
- Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. - PMC - PubMed
-
- Lafond K.E., Nair H., Rasooly M.H., Valente F., Booy R., Rahman M., Kitsutani P., Yu H., Guzman G., Coulibaly D., et al. Global Role and Burden of Influenza in Pediatric Respiratory Hospitalizations, 1982–2012: A Systematic Analysis. PLoS Med. 2016;13:e1001977. doi: 10.1371/journal.pmed.1001977. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

