Luteolin, a natural flavonoid, inhibits methylglyoxal induced apoptosis via the mTOR/4E-BP1 signaling pathway
- PMID: 28801605
- PMCID: PMC5554232
- DOI: 10.1038/s41598-017-08204-6
Luteolin, a natural flavonoid, inhibits methylglyoxal induced apoptosis via the mTOR/4E-BP1 signaling pathway
Abstract
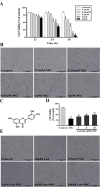
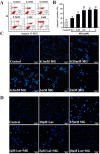
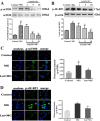
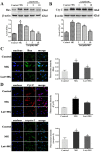
Methylglyoxal (MG) accumulation has been observed in human cerebrospinal fluid and body tissues under hyperglycaemic conditions. Recent research has demonstrated that MG-induces neuronal cell apoptosis, which promotes the development of diabetic encephalopathy. Our previous animal study has shown that luteolin, a natural flavonoid, attenuates diabetes-associated cognitive dysfunction. To further explore the neuroprotective properties of luteolin, we investigated the inhibitive effect of luteolin on MG-induced apoptosis in PC12 neuronal cells. We found that MG inhibited cell viability in a dose-dependent manner and induced apoptosis in PC12 cells. Pretreatment with Luteolin significantly elevated cell viability, reduced MG-induced apoptosis, inhibited the activation of the mTOR/4E-BP1 signaling pathway, and decreased pro-apoptotic proteins, Bax, Cytochrome C as well as caspase-3. Furthermore, we found that pretreatment with the mTOR inhibitor, rapamycin, significantly reduced the expression of the pro-apoptotic protein Bax. Therefore, these observations unambiguously suggest that the inhibitive effect of Luteolin against MG-induced apoptosis in PC12 cells is associated with inhibition of the mTOR/4E-BP1 signaling pathway.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Rotenone induction of hydrogen peroxide inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways, leading to neuronal apoptosis.Toxicol Sci. 2015 Jan;143(1):81-96. doi: 10.1093/toxsci/kfu211. Epub 2014 Oct 9. Toxicol Sci. 2015. PMID: 25304210 Free PMC article.
-
Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson's disease.Cell Signal. 2014 Aug;26(8):1680-1689. doi: 10.1016/j.cellsig.2014.04.009. Epub 2014 Apr 12. Cell Signal. 2014. PMID: 24726895 Free PMC article.
-
Neuroprotective effects of luteolin against apoptosis induced by 6-hydroxydopamine on rat pheochromocytoma PC12 cells.Pharm Biol. 2013 Feb;51(2):190-6. doi: 10.3109/13880209.2012.716852. Epub 2012 Oct 5. Pharm Biol. 2013. PMID: 23035972
-
Luteolin mediated targeting of protein network and microRNAs in different cancers: Focus on JAK-STAT, NOTCH, mTOR and TRAIL-mediated signaling pathways.Pharmacol Res. 2020 Oct;160:105188. doi: 10.1016/j.phrs.2020.105188. Epub 2020 Sep 9. Pharmacol Res. 2020. PMID: 32919041 Review.
-
Breast Cancer Apoptosis and the Therapeutic Role of Luteolin.Chirurgia (Bucur). 2021 Mar-Apr;116(2):170-177. doi: 10.21614/chirurgia.116.2.170. Chirurgia (Bucur). 2021. PMID: 33950812 Review.
Cited by
-
Luteolin prevents cadmium-induced PC12 cell death by suppressing the Akt/mTOR signaling pathway.Medicine (Baltimore). 2024 Nov 1;103(44):e40372. doi: 10.1097/MD.0000000000040372. Medicine (Baltimore). 2024. PMID: 39496018 Free PMC article.
-
Optimization of Extraction Process and Analysis of Biological Activity of Flavonoids from Leaves of Cultivated 'Qi-Nan' Agarwood.Molecules. 2024 Apr 17;29(8):1828. doi: 10.3390/molecules29081828. Molecules. 2024. PMID: 38675648 Free PMC article.
-
Autophagy Functions to Prevent Methylglyoxal-Induced Apoptosis in HK-2 Cells.Oxid Med Cell Longev. 2020 Jun 4;2020:8340695. doi: 10.1155/2020/8340695. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32566104 Free PMC article.
-
Noise Induced Depression-Like Behavior, Neuroinflammation and Synaptic Plasticity Impairments: The Protective Effects of Luteolin.Neurochem Res. 2022 Nov;47(11):3318-3330. doi: 10.1007/s11064-022-03683-0. Epub 2022 Aug 17. Neurochem Res. 2022. PMID: 35978229
-
The Impact of Oxidative Stress and AKT Pathway on Cancer Cell Functions and Its Application to Natural Products.Antioxidants (Basel). 2022 Sep 19;11(9):1845. doi: 10.3390/antiox11091845. Antioxidants (Basel). 2022. PMID: 36139919 Free PMC article. Review.
References
-
- Kuhad A, Chopra K. Neurobiology of diabetic encephalopathy. Drug Future. 2008;33:763–775. doi: 10.1358/dof.2008.033.09.1232462. - DOI
-
- Okouchi M, et al. Insulin protection against carbonyl and hyperglycemic stress-induced neuronal cell apoptosis. Diabetes. 2007;56:345–345.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

