Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism
- PMID: 28793934
- PMCID: PMC5550934
- DOI: 10.1186/s40168-017-0312-4
Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism
Abstract
Background: Statins are a class of therapeutics used to regulate serum cholesterol and reduce the risk of heart disease. Although statins are highly effective in removing cholesterol from the blood, their consumption has been linked to potential adverse effects in some individuals. The most common events associated with statin intolerance are myopathy and increased risk of developing type 2 diabetes mellitus. However, the pathological mechanism through which statins cause these adverse effects is not well understood.
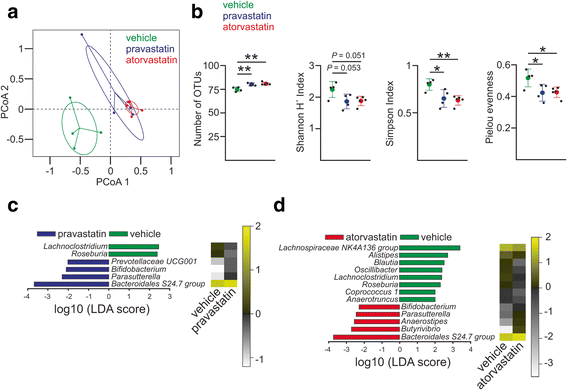
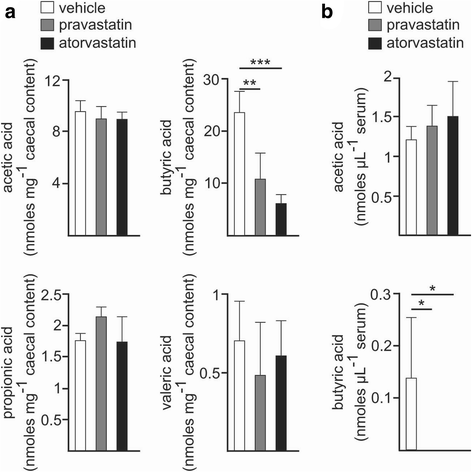
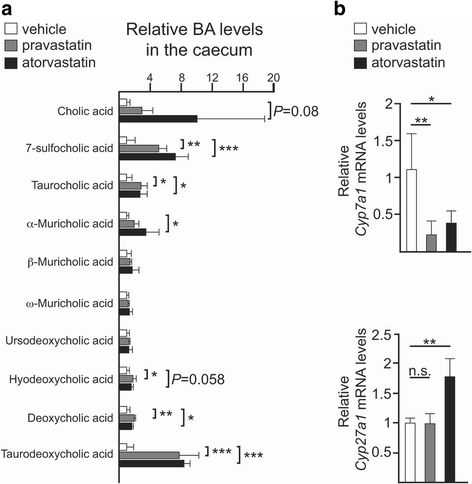
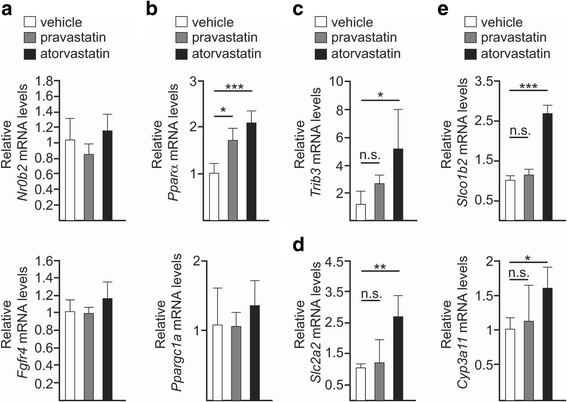
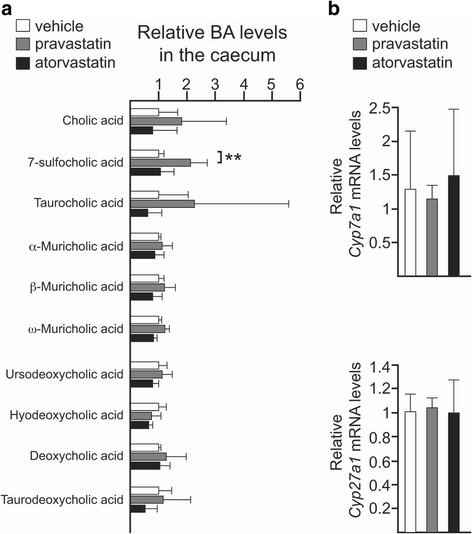
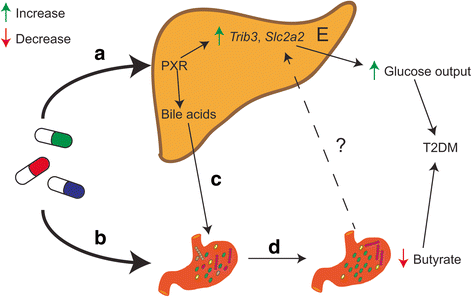
Results: Using a murine model, we describe for the first time profound changes in the microbial composition of the gut following statin treatment. This remodelling affected the diversity and metabolic profile of the gut microbiota and was associated with reduced production of butyrate. Statins altered both the size and composition of the bile acid pool in the intestine, tentatively explaining the observed gut dysbiosis. As also observed in patients, statin-treated mice trended towards increased fasting blood glucose levels and weight gain compared to controls. Statin treatment affected the hepatic expression of genes involved in lipid and glucose metabolism. Using gene knockout mice, we demonstrated that the observed effects were mediated through pregnane X receptor (PXR).
Conclusion: This study demonstrates that statin therapy drives a profound remodelling of the gut microbiota, hepatic gene deregulation and metabolic alterations in mice through a PXR-dependent mechanism. Since the demonstrated importance of the intestinal microbial community in host health, this work provides new perspectives to help prevent the statin-associated unintended metabolic effects.
Keywords: Bile acids; Dysbiosis; Gut microbiota; Pregnane X receptor; Short-chain fatty acids; Statins; Type 2 diabetes mellitus.
Conflict of interest statement
Ethics approval
Ethics approval was obtained from the Curtin University Animal Ethics Committee, and all procedures were in strict accordance with guidelines for the care and use of laboratory animals specified by this committee (reference number AEC-2014-36).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
The Role of the Gut Microbiota in Bile Acid Metabolism.Ann Hepatol. 2017 Nov;16(Suppl. 1: s3-105.):s15-s20. doi: 10.5604/01.3001.0010.5494. Ann Hepatol. 2017. PMID: 29080339 Review.
-
Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation.Free Radic Biol Med. 2017 Jan;102:188-202. doi: 10.1016/j.freeradbiomed.2016.11.037. Epub 2016 Nov 25. Free Radic Biol Med. 2017. PMID: 27890642
-
Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex.J Autoimmun. 2018 Aug;92:12-34. doi: 10.1016/j.jaut.2018.05.008. Epub 2018 Jun 1. J Autoimmun. 2018. PMID: 29861127 Review.
-
Gut microbiota, cirrhosis, and alcohol regulate bile acid metabolism in the gut.Dig Dis. 2015;33(3):338-45. doi: 10.1159/000371678. Epub 2015 May 27. Dig Dis. 2015. PMID: 26045267 Free PMC article.
-
The influence of rosuvastatin on the gastrointestinal microbiota and host gene expression profiles.Am J Physiol Gastrointest Liver Physiol. 2017 May 1;312(5):G488-G497. doi: 10.1152/ajpgi.00149.2016. Epub 2017 Feb 16. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 28209601
Cited by
-
Morchella esculenta mushroom polysaccharide attenuates diabetes and modulates intestinal permeability and gut microbiota in a type 2 diabetic mice model.Front Nutr. 2022 Oct 6;9:984695. doi: 10.3389/fnut.2022.984695. eCollection 2022. Front Nutr. 2022. PMID: 36276816 Free PMC article.
-
Gut Microbiota Modulation as a Novel Therapeutic Strategy in Cardiometabolic Diseases.Foods. 2022 Aug 25;11(17):2575. doi: 10.3390/foods11172575. Foods. 2022. PMID: 36076760 Free PMC article. Review.
-
Dietary metabolism, the gut microbiome, and heart failure.Nat Rev Cardiol. 2019 Mar;16(3):137-154. doi: 10.1038/s41569-018-0108-7. Nat Rev Cardiol. 2019. PMID: 30410105 Free PMC article. Review.
-
Simvastatin Therapy in Multiple Sclerosis Patients with Respect to Gut Microbiome-Friend or Foe?J Neuroimmune Pharmacol. 2019 Dec;14(4):531-533. doi: 10.1007/s11481-019-09881-y. Epub 2019 Oct 18. J Neuroimmune Pharmacol. 2019. PMID: 31628587 No abstract available.
-
Ameliorative Effects of Lactobacillus paracasei L14 on Oxidative Stress and Gut Microbiota in Type 2 Diabetes Mellitus Rats.Antioxidants (Basel). 2023 Jul 28;12(8):1515. doi: 10.3390/antiox12081515. Antioxidants (Basel). 2023. PMID: 37627510 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

