Biocompatibility of Advanced Manufactured Titanium Implants-A Review
- PMID: 28788296
- PMCID: PMC5456424
- DOI: 10.3390/ma7128168
Biocompatibility of Advanced Manufactured Titanium Implants-A Review
Abstract
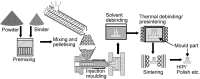
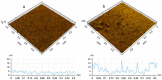
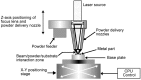
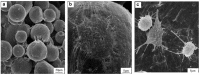
Titanium (Ti) and its alloys may be processed via advanced powder manufacturing routes such as additive layer manufacturing (or 3D printing) or metal injection moulding. This field is receiving increased attention from various manufacturing sectors including the medical devices sector. It is possible that advanced manufacturing techniques could replace the machining or casting of metal alloys in the manufacture of devices because of associated advantages that include design flexibility, reduced processing costs, reduced waste, and the opportunity to more easily manufacture complex or custom-shaped implants. The emerging advanced manufacturing approaches of metal injection moulding and additive layer manufacturing are receiving particular attention from the implant fabrication industry because they could overcome some of the difficulties associated with traditional implant fabrication techniques such as titanium casting. Using advanced manufacturing, it is also possible to produce more complex porous structures with improved mechanical performance, potentially matching the modulus of elasticity of local bone. While the economic and engineering potential of advanced manufacturing for the manufacture of musculo-skeletal implants is therefore clear, the impact on the biocompatibility of the materials has been less investigated. In this review, the capabilities of advanced powder manufacturing routes in producing components that are suitable for biomedical implant applications are assessed with emphasis placed on surface finishes and porous structures. Given that biocompatibility and host bone response are critical determinants of clinical performance, published studies of in vitro and in vivo research have been considered carefully. The review concludes with a future outlook on advanced Ti production for biomedical implants using powder metallurgy.
Keywords: 3-D printing; CP-Ti; Ti6Al4V; additive manufacturing; biocompatibility; cytotoxicity; implants; metal injection moulding; powder metallurgy; titanium.
Conflict of interest statement
The author declares no conflict of interest.
Figures

Similar articles
-
Additive manufacturing of titanium alloys in the biomedical field: processes, properties and applications.J Appl Biomater Funct Mater. 2018 Apr;16(2):57-67. doi: 10.5301/jabfm.5000371. J Appl Biomater Funct Mater. 2018. PMID: 28967051 Review.
-
A comprehensive review on metallic implant biomaterials and their subtractive manufacturing.Int J Adv Manuf Technol. 2022;120(3-4):1473-1530. doi: 10.1007/s00170-022-08770-8. Epub 2022 Feb 23. Int J Adv Manuf Technol. 2022. PMID: 35228769 Free PMC article. Review.
-
Osteoconductivity of bioactive Ti-6Al-4V implants with lattice-shaped interconnected large pores fabricated by electron beam melting.J Biomater Appl. 2021 Apr;35(9):1153-1167. doi: 10.1177/0885328220968218. Epub 2020 Oct 26. J Biomater Appl. 2021. PMID: 33106079
-
Rational design, bio-functionalization and biological performance of hybrid additive manufactured titanium implants for orthopaedic applications: A review.J Mech Behav Biomed Mater. 2020 May;105:103671. doi: 10.1016/j.jmbbm.2020.103671. Epub 2020 Feb 6. J Mech Behav Biomed Mater. 2020. PMID: 32090892 Review.
-
A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical applications.Biodes Manuf. 2022;5(2):371-395. doi: 10.1007/s42242-021-00170-3. Epub 2021 Oct 26. Biodes Manuf. 2022. PMID: 34721937 Free PMC article. Review.
Cited by
-
Laminin Adsorption and Adhesion of Neurons and Glial Cells on Carbon Implanted Titania Nanotube Scaffolds for Neural Implant Applications.Nanomaterials (Basel). 2022 Nov 1;12(21):3858. doi: 10.3390/nano12213858. Nanomaterials (Basel). 2022. PMID: 36364633 Free PMC article.
-
Crystal Structure Evolution, Microstructure Formation, and Properties of Mechanically Alloyed Ultrafine-Grained Ti-Zr-Nb Alloys at 36≤Ti≤70 (at. %).Materials (Basel). 2020 Jan 27;13(3):587. doi: 10.3390/ma13030587. Materials (Basel). 2020. PMID: 32012767 Free PMC article.
-
Evaluation of the osteogenesis and osseointegration of titanium alloys coated with graphene: an in vivo study.Sci Rep. 2018 Jan 30;8(1):1843. doi: 10.1038/s41598-018-19742-y. Sci Rep. 2018. PMID: 29382859 Free PMC article.
-
Polydopamine coating promotes early osteogenesis in 3D printing porous Ti6Al4V scaffolds.Ann Transl Med. 2019 Jun;7(11):240. doi: 10.21037/atm.2019.04.79. Ann Transl Med. 2019. PMID: 31317010 Free PMC article.
-
Biocompatible Materials Based on Self-Assembling Peptides on Ti25Nb10Zr Alloy: Molecular Structure and Organization Investigated by Synchrotron Radiation Induced Techniques.Nanomaterials (Basel). 2018 Mar 7;8(3):148. doi: 10.3390/nano8030148. Nanomaterials (Basel). 2018. PMID: 29518968 Free PMC article.
References
-
- Elias C.N., Lima J.H.C., Valiev R., Meyers M.A. Biomedical applications of titanium and its alloys. JOM. 2008;60:46–49.
-
- BomBač D., Brojan M., Fajfar P., Kosel F., Turk R. Review of materials in medical applications. RMZ Mater. Geoenviron. 2007;54:471–499.
-
- Mueller E., Kammula R., Marlowe D. Regulation of biomaterials and medical devices. MRS Bull. 1991;16:39–41. doi: 10.1557/S0883769400056037. - DOI
-
- Froes F.H. Titanium powder metallurgy: A review—Part 1. Adv. Mater. Process. 2012;170:16–22.
-
- Guo S., Qu X., He X., Zhou T., Duan B. Powder injection molding of Ti-6Al-4V alloy. J. Mater. Process. Technol. 2006;173:310–314. doi: 10.1016/j.jmatprotec.2005.12.001. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

