Poxviruses Utilize Multiple Strategies to Inhibit Apoptosis
- PMID: 28786952
- PMCID: PMC5580472
- DOI: 10.3390/v9080215
Poxviruses Utilize Multiple Strategies to Inhibit Apoptosis
Abstract
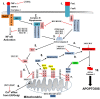
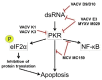
Cells have multiple means to induce apoptosis in response to viral infection. Poxviruses must prevent activation of cellular apoptosis to ensure successful replication. These viruses devote a substantial portion of their genome to immune evasion. Many of these immune evasion products expressed during infection antagonize cellular apoptotic pathways. Poxvirus products target multiple points in both the extrinsic and intrinsic apoptotic pathways, thereby mitigating apoptosis during infection. Interestingly, recent evidence indicates that poxviruses also hijack cellular means of eliminating apoptotic bodies as a means to spread cell to cell through a process called apoptotic mimicry. Poxviruses are the causative agent of many human and veterinary diseases. Further, there is substantial interest in developing these viruses as vectors for a variety of uses including vaccine delivery and as oncolytic viruses to treat certain human cancers. Therefore, an understanding of the molecular mechanisms through which poxviruses regulate the cellular apoptotic pathways remains a top research priority. In this review, we consider anti-apoptotic strategies of poxviruses focusing on three relevant poxvirus genera: Orthopoxvirus, Molluscipoxvirus, and Leporipoxvirus. All three genera express multiple products to inhibit both extrinsic and intrinsic apoptotic pathways with many of these products required for virulence.
Keywords: Molluscum Contagiosum Virus; Myxoma Virus; Vaccinia Virus; apoptosis; caspase; host defense; immune evasion; mitochondrial membrane permeabilization; poxvirus; protein kinase R.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Poxvirus host range genes.Adv Virus Res. 2008;71:135-71. doi: 10.1016/S0065-3527(08)00003-1. Adv Virus Res. 2008. PMID: 18585528 Review.
-
Poxviruses and immune evasion.Annu Rev Immunol. 2003;21:377-423. doi: 10.1146/annurev.immunol.21.120601.141049. Epub 2001 Dec 19. Annu Rev Immunol. 2003. PMID: 12543935 Review.
-
Poxviruses and the evolution of host range and virulence.Infect Genet Evol. 2014 Jan;21:15-40. doi: 10.1016/j.meegid.2013.10.014. Epub 2013 Oct 24. Infect Genet Evol. 2014. PMID: 24161410 Free PMC article. Review.
-
Virulent Poxviruses Inhibit DNA Sensing by Preventing STING Activation.J Virol. 2018 Apr 27;92(10):e02145-17. doi: 10.1128/JVI.02145-17. Print 2018 May 15. J Virol. 2018. PMID: 29491158 Free PMC article.
-
Poxvirus Safety Analysis in the Pregnant Mouse Model, Vaccinia, and Raccoonpox Viruses.Methods Mol Biol. 2017;1581:121-129. doi: 10.1007/978-1-4939-6869-5_7. Methods Mol Biol. 2017. PMID: 28374246
Cited by
-
Mastering Death: The Roles of Viral Bcl-2 in dsDNA Viruses.Viruses. 2024 May 30;16(6):879. doi: 10.3390/v16060879. Viruses. 2024. PMID: 38932171 Free PMC article. Review.
-
MicroRNAs as immune regulators and biomarkers in tuberculosis.Front Immunol. 2022 Oct 27;13:1027472. doi: 10.3389/fimmu.2022.1027472. eCollection 2022. Front Immunol. 2022. PMID: 36389769 Free PMC article. Review.
-
SARS-CoV-2 encoded microRNAs are involved in the process of virus infection and host immune response.J Biomed Res. 2021 Jan 29;35(3):216-227. doi: 10.7555/JBR.35.20200154. J Biomed Res. 2021. PMID: 33963094 Free PMC article.
-
Monkeypox: disease epidemiology, host immunity and clinical interventions.Nat Rev Immunol. 2022 Oct;22(10):597-613. doi: 10.1038/s41577-022-00775-4. Epub 2022 Sep 5. Nat Rev Immunol. 2022. PMID: 36064780 Free PMC article. Review.
-
Evolutionary Profile for (Host and Viral) MLKL Indicates Its Activities as a Battlefront for Extensive Counteradaptation.Mol Biol Evol. 2021 Dec 9;38(12):5405-5422. doi: 10.1093/molbev/msab256. Mol Biol Evol. 2021. PMID: 34436583 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

