A brain proteomic investigation of rapamycin effects in the Tsc1+/- mouse model
- PMID: 28775826
- PMCID: PMC5540199
- DOI: 10.1186/s13229-017-0151-y
A brain proteomic investigation of rapamycin effects in the Tsc1+/- mouse model
Abstract
Background: Tuberous sclerosis complex (TSC) is a rare monogenic disorder characterized by benign tumors in multiple organs as well as a high prevalence of epilepsy, intellectual disability and autism. TSC is caused by inactivating mutations in the TSC1 or TSC2 genes. Heterozygocity induces hyperactivation of mTOR which can be inhibited by mTOR inhibitors, such as rapamycin, which have proven efficacy in the treatment of TSC-associated symptoms. The aim of the present study was (1) to identify molecular changes associated with social and cognitive deficits in the brain tissue of Tsc1+/- mice and (2) to investigate the molecular effects of rapamycin treatment, which has been shown to ameliorate genotype-related behavioural deficits.
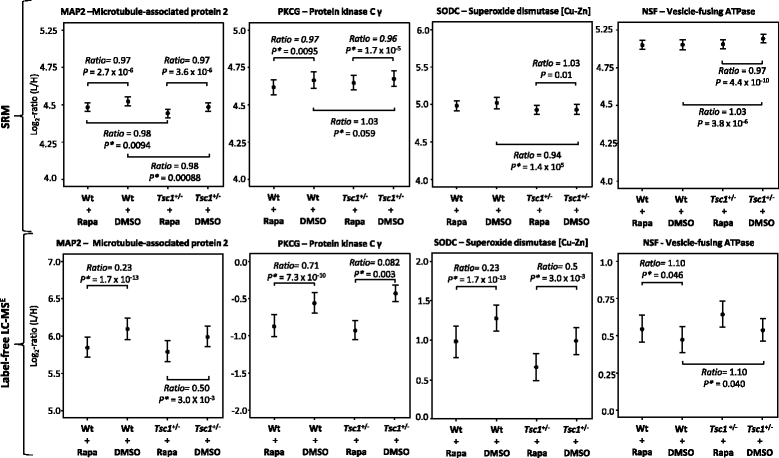
Methods: Molecular alterations in the frontal cortex and hippocampus of Tsc1+/- and control mice, with or without rapamycin treatment, were investigated. A quantitative mass spectrometry-based shotgun proteomic approach (LC-MSE) was employed as an unbiased method to detect changes in protein levels. Changes identified in the initial profiling stage were validated using selected reaction monitoring (SRM). Protein Set Enrichment Analysis was employed to identify dysregulated pathways.
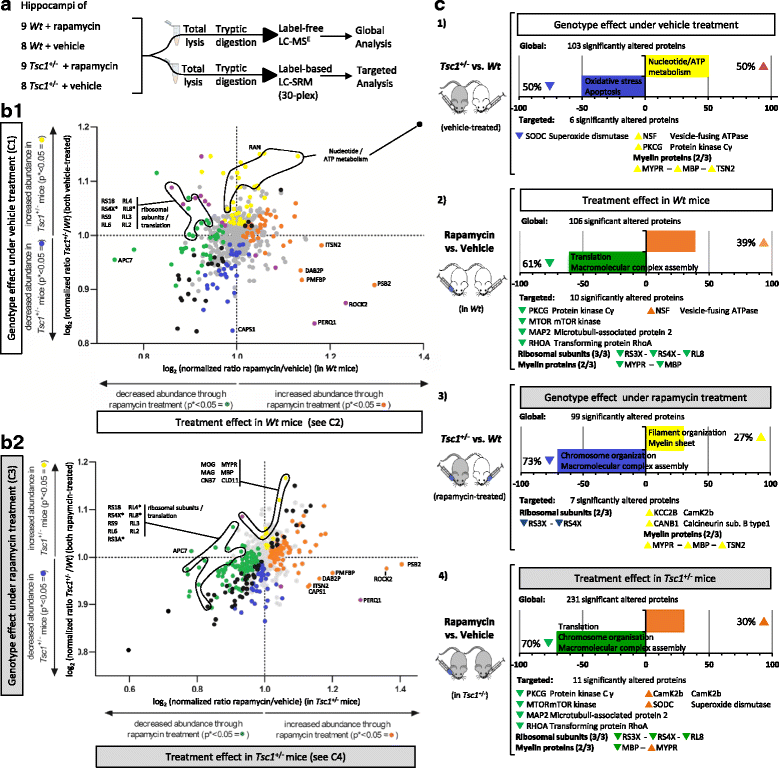
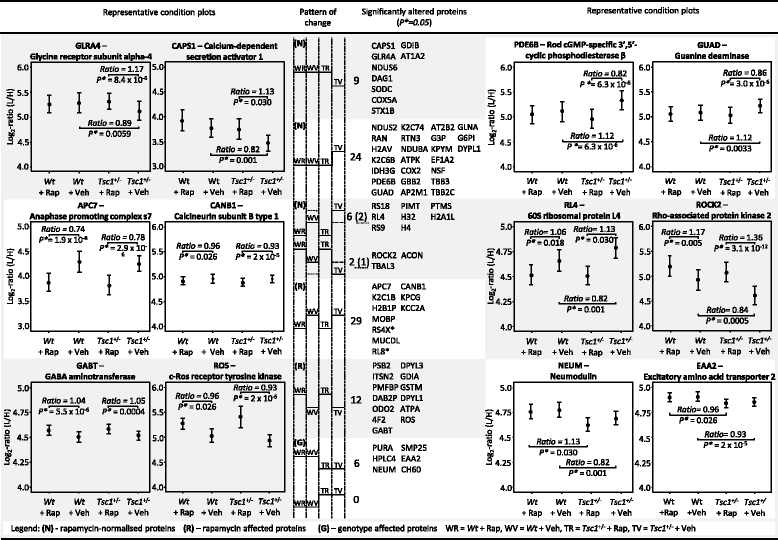
Results: LC-MSE analysis of Tsc1+/- mice and controls (n = 30) identified 51 proteins changed in frontal cortex and 108 in the hippocampus. Bioinformatic analysis combined with targeted proteomic validation revealed several dysregulated molecular pathways. Using targeted assays, proteomic alterations in the hippocampus validated the pathways "myelination", "dendrite," and "oxidative stress", an upregulation of ribosomal proteins and the mTOR kinase. LC-MSE analysis was also employed on Tsc1+/- and wildtype mice (n = 34) treated with rapamycin or vehicle. Rapamycin treatment exerted a stronger proteomic effect in Tsc1+/- mice with significant changes (mainly decreased expression) in 231 and 106 proteins, respectively. The cellular pathways "oxidative stress" and "apoptosis" were found to be affected in Tsc1+/- mice and the cellular compartments "myelin sheet" and "neurofilaments" were affected by rapamycin treatment. Thirty-three proteins which were altered in Tsc1+/- mice were normalized following rapamycin treatment, amongst them oxidative stress related proteins, myelin-specific and ribosomal proteins.
Conclusions: Molecular changes in the Tsc1+/- mouse brain were more prominent in the hippocampus compared to the frontal cortex. Pathways linked to myelination and oxidative stress response were prominently affected and, at least in part, normalized following rapamycin treatment. The results could aid in the identification of novel drug targets for the treatment of cognitive, social and psychiatric symptoms in autism spectrum disorders. Similar pathways have also been implicated in other psychiatric and neurodegenerative disorders and could imply similar disease processes. Thus, the potential efficacy of mTOR inhibitors warrants further investigation not only for autism spectrum disorders but also for other neuropsychiatric and neurodegenerative diseases.
Keywords: Animal model; Proteomics; Rapamycin; SRM; Tuberous sclerosis.
Conflict of interest statement
Ethics approval and consent to participate
All animal experiments were approved by the Dutch Ethical Committee or in accordance with Institutional Animal Care and Use Committee guidelines.
All animal experiments were approved by the Dutch Animal Experiment Committee (Dierexperimenten commissie [DEC]) and in accordance with Dutch animal care and use laws.
Consent for publication
Not applicable.
Competing interests
S.B. is a director of Psynova Neurotech Ltd. and PsyOmics Ltd. The other authors declare no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Proteomic analysis of murine Tsc1-deficient neural stem progenitor cells.J Proteomics. 2023 Jul 15;283-284:104928. doi: 10.1016/j.jprot.2023.104928. Epub 2023 May 18. J Proteomics. 2023. PMID: 37207814
-
Tsc2 mutation rather than Tsc1 mutation dominantly causes a social deficit in a mouse model of tuberous sclerosis complex.Hum Genomics. 2023 Feb 2;17(1):4. doi: 10.1186/s40246-023-00450-2. Hum Genomics. 2023. PMID: 36732866 Free PMC article.
-
Translatome analysis of tuberous sclerosis complex 1 patient-derived neural progenitor cells reveals rapamycin-dependent and independent alterations.Mol Autism. 2023 Oct 25;14(1):39. doi: 10.1186/s13229-023-00572-3. Mol Autism. 2023. PMID: 37880800 Free PMC article.
-
A circuitry and biochemical basis for tuberous sclerosis symptoms: from epilepsy to neurocognitive deficits.Int J Dev Neurosci. 2013 Nov;31(7):667-78. doi: 10.1016/j.ijdevneu.2013.02.008. Epub 2013 Feb 26. Int J Dev Neurosci. 2013. PMID: 23485365 Free PMC article. Review.
-
Differentiating the mTOR inhibitors everolimus and sirolimus in the treatment of tuberous sclerosis complex.Neuro Oncol. 2015 Dec;17(12):1550-9. doi: 10.1093/neuonc/nov152. Epub 2015 Aug 19. Neuro Oncol. 2015. PMID: 26289591 Free PMC article. Review.
Cited by
-
The molecular genetics of PI3K/PTEN/AKT/mTOR pathway in the malformations of cortical development.Genes Dis. 2023 Jul 16;11(5):101021. doi: 10.1016/j.gendis.2023.04.041. eCollection 2024 Sep. Genes Dis. 2023. PMID: 39006182 Free PMC article. Review.
-
Autism spectrum disorder: pathogenesis, biomarker, and intervention therapy.MedComm (2020). 2024 Mar 2;5(3):e497. doi: 10.1002/mco2.497. eCollection 2024 Mar. MedComm (2020). 2024. PMID: 38434761 Free PMC article. Review.
-
Brain somatic mutations in MTOR reveal translational dysregulations underlying intractable focal epilepsy.J Clin Invest. 2019 Oct 1;129(10):4207-4223. doi: 10.1172/JCI127032. J Clin Invest. 2019. PMID: 31483294 Free PMC article.
-
Abnormal activation of Yap/Taz contributes to the pathogenesis of tuberous sclerosis complex.Hum Mol Genet. 2022 Jun 22;31(12):1979-1996. doi: 10.1093/hmg/ddab374. Hum Mol Genet. 2022. PMID: 34999833 Free PMC article.
-
PKC downregulation upon rapamycin treatment attenuates mitochondrial disease.Nat Metab. 2020 Dec;2(12):1472-1481. doi: 10.1038/s42255-020-00319-x. Epub 2020 Dec 14. Nat Metab. 2020. PMID: 33324011 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

