Intramolecular Interactions Overcome Hydration to Drive the Collapse Transition of Gly15
- PMID: 28774177
- PMCID: PMC5912879
- DOI: 10.1021/acs.jpcb.7b05469
Intramolecular Interactions Overcome Hydration to Drive the Collapse Transition of Gly15
Abstract
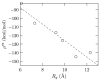
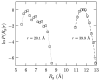
Simulations and experiments show oligo-glycines, polypeptides lacking any side chains, can collapse in water. We assess the hydration thermodynamics of this collapse by calculating the hydration free energy at each of the end points of the reaction coordinate, here taken as the end-to-end distance (r) in the chain. To examine the role of the various conformations for a given r, we study the conditional distribution, P(Rg|r), of the radius of gyration for a given value of r. The free energy change versus Rg, -kBT ln P(Rg|r), is found to vary more gently compared to the corresponding variation in the excess hydration free energy. Using this observation within a multistate generalization of the potential distribution theorem, we calculate a tight upper bound for the hydration free energy of the peptide for a given r. On this basis, we find that peptide hydration greatly favors the expanded state of the chain, despite primitive hydrophobic effects favoring chain collapse. The net free energy of collapse is seen to be a delicate balance between opposing intrapeptide and hydration effects, with intrapeptide contributions favoring collapse.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures


Similar articles
-
Importance of Hydrophilic Hydration and Intramolecular Interactions in the Thermodynamics of Helix-Coil Transition and Helix-Helix Assembly in a Deca-Alanine Peptide.J Phys Chem B. 2016 Jan 14;120(1):69-76. doi: 10.1021/acs.jpcb.5b09881. Epub 2015 Dec 22. J Phys Chem B. 2016. PMID: 26649757 Free PMC article.
-
Conditional solvation thermodynamics of isoleucine in model peptides and the limitations of the group-transfer model.J Phys Chem B. 2014 Apr 17;118(15):4080-7. doi: 10.1021/jp500727u. Epub 2014 Apr 3. J Phys Chem B. 2014. PMID: 24650057 Free PMC article.
-
Peptide backbone effect on hydration free energies of amino acid side chains.J Phys Chem B. 2014 Nov 20;118(46):13162-8. doi: 10.1021/jp5094146. Epub 2014 Nov 5. J Phys Chem B. 2014. PMID: 25338222
-
Protein collapse driven against solvation free energy without H-bonds.Protein Sci. 2016 Jan;25(1):103-10. doi: 10.1002/pro.2749. Epub 2015 Aug 8. Protein Sci. 2016. PMID: 26174309 Free PMC article.
-
Distinct role of hydration water in protein misfolding and aggregation revealed by fluctuating thermodynamics analysis.Acc Chem Res. 2015 Apr 21;48(4):956-65. doi: 10.1021/acs.accounts.5b00032. Epub 2015 Apr 6. Acc Chem Res. 2015. PMID: 25844814 Review.
Cited by
-
Relationship of Sequence and Phase Separation in Protein Low-Complexity Regions.Biochemistry. 2018 May 1;57(17):2478-2487. doi: 10.1021/acs.biochem.8b00008. Epub 2018 Mar 16. Biochemistry. 2018. PMID: 29517898 Free PMC article.
-
Improvements to the ABSINTH Force Field for Proteins Based on Experimentally Derived Amino Acid Specific Backbone Conformational Statistics.J Chem Theory Comput. 2019 Feb 12;15(2):1367-1382. doi: 10.1021/acs.jctc.8b00573. Epub 2019 Jan 22. J Chem Theory Comput. 2019. PMID: 30633502 Free PMC article.
-
Pearl-Necklace-Like Local Ordering Drives Polypeptide Collapse.Macromolecules. 2019 Aug 13;52(15):5491-5498. doi: 10.1021/acs.macromol.9b00562. Epub 2019 Jul 15. Macromolecules. 2019. PMID: 31631912 Free PMC article.
-
Sequence determinants of in cell condensate morphology, dynamics, and oligomerization as measured by number and brightness analysis.Cell Commun Signal. 2021 Jun 5;19(1):65. doi: 10.1186/s12964-021-00744-9. Cell Commun Signal. 2021. PMID: 34090478 Free PMC article.
-
Glycine-Rich Peptides from FUS Have an Intrinsic Ability to Self-Assemble into Fibers and Networked Fibrils.Biochemistry. 2021 Nov 2;60(43):3213-3222. doi: 10.1021/acs.biochem.1c00501. Epub 2021 Oct 14. Biochemistry. 2021. PMID: 34648275 Free PMC article.
References
-
- Kauzmann W. Some Factors in the Interpretation of Protein Denaturation. Adv Protein Chem. 1959;14:1–63. - PubMed
-
- Chandler D. Interfaces and the Driving Force of Hydrophobic Assembly. Nature. 2005;437:640–647. - PubMed
-
- Dill KA. Dominant Forces in Protein Folding. Biochemistry. 1990;29:7133–7155. - PubMed
-
- Dill KA, MacCallum JL. The Protein-Folding Problem, 50 Years On. Science. 2012;338:1042–1046. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

