Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse
- PMID: 28769083
- PMCID: PMC5541101
- DOI: 10.1038/s41598-017-07672-0
Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse
Abstract
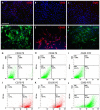
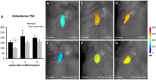
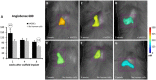
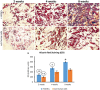
Traumatic injury or surgical excision of diseased bone tissue usually require the reconstruction of large bone defects unable to heal spontaneously, especially in older individuals. This is a big challenge requiring the development of biomaterials mimicking the bone structure and capable of inducing the right commitment of cells seeded within the scaffold. In particular, given their properties and large availability, the human adipose-derived stem cells are considered as the better candidate for autologous cell transplantation. In order to evaluate the regenerative potential of these cells along with an osteoinductive biomaterial, we have used collagen/hydroxyapatite scaffolds to test ectopic bone formation after subcutaneous implantation in mice. The process was analysed both in vivo, by Fluorescent Molecular Tomography (FMT), and ex vivo, to evaluate the formation of bone and vascular structures. The results have shown that the biomaterial could itself be able of promoting differentiation of host cells and bone formation, probably by means of its intrinsic chemical and structural properties, namely the microenvironment. However, when charged with human mesenchymal stem cells, the ectopic bone formation within the scaffold was increased. We believe that these results represent an important advancement in the field of bone physiology, as well as in regenerative medicine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Fractionated human adipose tissue as a native biomaterial for the generation of a bone organ by endochondral ossification.Acta Biomater. 2018 Sep 1;77:142-154. doi: 10.1016/j.actbio.2018.07.004. Epub 2018 Jul 4. Acta Biomater. 2018. PMID: 30126590
-
Adipose mesenchymal stem cells associated with xenograft in a guided bone regeneration model: a histomorphometric study in rabbit calvaria.Int J Oral Maxillofac Implants. 2015 Nov-Dec;30(6):1415-22. doi: 10.11607/jomi.4164. Int J Oral Maxillofac Implants. 2015. PMID: 26574866
-
Bone regeneration from human mesenchymal stem cells on porous hydroxyapatite-PLGA-collagen bioactive polymer scaffolds.Biomed Mater Eng. 2017;28(6):671-685. doi: 10.3233/BME-171703. Biomed Mater Eng. 2017. PMID: 29171970
-
Recent Advances in Scaffold Design and Material for Vascularized Tissue-Engineered Bone Regeneration.Adv Healthc Mater. 2019 May;8(10):e1801433. doi: 10.1002/adhm.201801433. Epub 2019 Apr 2. Adv Healthc Mater. 2019. PMID: 30938094 Review.
-
Adipose-derived mesenchymal cells for bone regereneration: state of the art.Biomed Res Int. 2013;2013:416391. doi: 10.1155/2013/416391. Epub 2013 Nov 7. Biomed Res Int. 2013. PMID: 24307997 Free PMC article. Review.
Cited by
-
Ageing attenuates bone healing by mesenchymal stem cells in a microribbon hydrogel with a murine long bone critical-size defect model.Immun Ageing. 2022 Mar 12;19(1):14. doi: 10.1186/s12979-022-00272-1. Immun Ageing. 2022. PMID: 35279175 Free PMC article.
-
Analysis of the miRNA and mRNA involved in osteogenesis of adipose-derived mesenchymal stem cells.Exp Ther Med. 2018 Aug;16(2):1111-1120. doi: 10.3892/etm.2018.6303. Epub 2018 Jun 13. Exp Ther Med. 2018. PMID: 30116362 Free PMC article.
-
PCL/Col I-based magnetic nanocomposite scaffold provides an osteoinductive environment for ADSCs in osteogenic cues-free media conditions.Stem Cell Res Ther. 2022 Apr 4;13(1):143. doi: 10.1186/s13287-022-02816-0. Stem Cell Res Ther. 2022. PMID: 35379318 Free PMC article.
-
Current evidence on potential of adipose derived stem cells to enhance bone regeneration and future projection.World J Stem Cells. 2021 Sep 26;13(9):1248-1277. doi: 10.4252/wjsc.v13.i9.1248. World J Stem Cells. 2021. PMID: 34630861 Free PMC article. Review.
-
In Vivo Evaluation of Biocompatibility and Chondrogenic Potential of a Cell-Free Collagen-Based Scaffold.Front Physiol. 2017 Nov 29;8:984. doi: 10.3389/fphys.2017.00984. eCollection 2017. Front Physiol. 2017. PMID: 29238307 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

