Bayesian Model Averaging with Change Points to Assess the Impact of Vaccination and Public Health Interventions
- PMID: 28767518
- PMCID: PMC5617796
- DOI: 10.1097/EDE.0000000000000719
Bayesian Model Averaging with Change Points to Assess the Impact of Vaccination and Public Health Interventions
Abstract
Background: Pneumococcal conjugate vaccines (PCVs) prevent invasive pneumococcal disease and pneumonia. However, some low-and middle-income countries have yet to introduce PCV into their immunization programs due, in part, to lack of certainty about the potential impact. Assessing PCV benefits is challenging because specific data on pneumococcal disease are often lacking, and it can be difficult to separate the effects of factors other than the vaccine that could also affect pneumococcal disease rates.
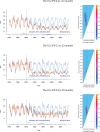
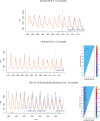
Methods: We assess PCV impact by combining Bayesian model averaging with change-point models to estimate the timing and magnitude of vaccine-associated changes, while controlling for seasonality and other covariates. We applied our approach to monthly time series of age-stratified hospitalizations related to pneumococcal infection in children younger 5 years of age in the United States, Brazil, and Chile.
Results: Our method accurately detected changes in data in which we knew true and noteworthy changes occurred, i.e., in simulated data and for invasive pneumococcal disease. Moreover, 24 months after the vaccine introduction, we detected reductions of 14%, 9%, and 9% in the United States, Brazil, and Chile, respectively, in all-cause pneumonia (ACP) hospitalizations for age group 0 to <1 years of age.
Conclusions: Our approach provides a flexible and sensitive method to detect changes in disease incidence that occur after the introduction of a vaccine or other intervention, while avoiding biases that exist in current approaches to time-trend analyses.
Conflict of interest statement
D.M.W. had received an investigator-initiated research grant from Pfizer and consulting fees from Pfizer, Merck, and Affinivax. The other authors have no conflicts to report.
Figures
Similar articles
-
Evaluating the impact of PCV-10 on invasive pneumococcal disease in Brazil: A time-series analysis.Hum Vaccin Immunother. 2016;12(2):285-92. doi: 10.1080/21645515.2015.1117713. Hum Vaccin Immunother. 2016. PMID: 26905679 Free PMC article.
-
Sinusitis and pneumonia hospitalization after introduction of pneumococcal conjugate vaccine.Pediatrics. 2014 Dec;134(6):e1528-36. doi: 10.1542/peds.2013-4177. Epub 2014 Nov 10. Pediatrics. 2014. PMID: 25384486
-
Impact of pneumococcal conjugate vaccine in children morbidity and mortality in Peru: Time series analyses.Vaccine. 2016 Sep 7;34(39):4738-4743. doi: 10.1016/j.vaccine.2016.07.027. Epub 2016 Aug 9. Vaccine. 2016. PMID: 27521230
-
Use of the 13-valent pneumococcal conjugate vaccine in children and adolescents aged 6 - 17 years.Expert Opin Biol Ther. 2013 Oct;13(10):1451-65. doi: 10.1517/14712598.2013.824419. Epub 2013 Jul 29. Expert Opin Biol Ther. 2013. PMID: 23889554 Review.
-
Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: review of evidence on indirect effects.Vaccine. 2013 Dec 17;32(1):133-45. doi: 10.1016/j.vaccine.2013.05.005. Epub 2013 May 16. Vaccine. 2013. PMID: 23684824 Review.
Cited by
-
The State of Use and Utility of Negative Controls in Pharmacoepidemiologic Studies.Am J Epidemiol. 2024 Feb 5;193(3):426-453. doi: 10.1093/aje/kwad201. Am J Epidemiol. 2024. PMID: 37851862 Free PMC article. Review.
-
Bayesian Model Averaging to Account for Model Uncertainty in Estimates of a Vaccine's Effectiveness.Clin Epidemiol. 2022 Oct 18;14:1167-1175. doi: 10.2147/CLEP.S378039. eCollection 2022. Clin Epidemiol. 2022. PMID: 36281232 Free PMC article.
-
Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California.Nat Med. 2022 Sep;28(9):1933-1943. doi: 10.1038/s41591-022-01887-z. Epub 2022 Jun 8. Nat Med. 2022. PMID: 35675841 Free PMC article.
-
Pulse oximetry and oxygen services for the care of children with pneumonia attending frontline health facilities in Lagos, Nigeria (INSPIRING-Lagos): study protocol for a mixed-methods evaluation.BMJ Open. 2022 May 2;12(5):e058901. doi: 10.1136/bmjopen-2021-058901. BMJ Open. 2022. PMID: 35501079 Free PMC article.
-
Impact after 10-year use of pneumococcal conjugate vaccine in the Brazilian national immunization program: an updated systematic literature review from 2015 to 2020.Hum Vaccin Immunother. 2022 Dec 31;18(1):1879578. doi: 10.1080/21645515.2021.1879578. Epub 2021 Mar 18. Hum Vaccin Immunother. 2022. PMID: 33735585 Free PMC article.
References
-
- O’Brien KL, Wolfson LJ, Watt JP, et al. ; Hib and Pneumococcal Global Burden of Disease Study Team. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902.. - PubMed
-
- Whitney CG, Farley MM, Hadler J, et al. ; Active Bacterial Core Surveillance of the Emerging Infections Program Network. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746.. - PubMed
-
- Harboe ZB, Dalby T, Weinberger DM, et al. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis. 2014;59:1066–1073.. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

