Mitochondrial function requires NGLY1
- PMID: 28750948
- PMCID: PMC6038697
- DOI: 10.1016/j.mito.2017.07.008
Mitochondrial function requires NGLY1
Abstract
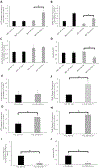
Mitochondrial respiratory chain (RC) diseases and congenital disorders of glycosylation (CDG) share extensive clinical overlap but are considered to have distinct cellular pathophysiology. Here, we demonstrate that an essential physiologic connection exists between cellular N-linked deglycosylation capacity and mitochondrial function. Following identification of altered muscle and liver mitochondrial amount and function in two children with a CDG subtype caused by NGLY1 deficiency, we evaluated mitochondrial physiology in NGLY1 disease human fibroblasts, and in NGLY1-knockout mouse embryonic fibroblasts and C. elegans. Across these distinct evolutionary models of cytosolic NGLY1 deficiency, a consistent disruption of mitochondrial physiology was present involving modestly reduced mitochondrial content with more pronounced impairment of mitochondrial membrane potential, increased mitochondrial matrix oxidant burden, and reduced cellular respiratory capacity. Lentiviral rescue restored NGLY1 expression and mitochondrial physiology in human and mouse fibroblasts, confirming that NGLY1 directly influences mitochondrial function. Overall, cellular deglycosylation capacity is shown to be a significant factor in mitochondrial RC disease pathogenesis across divergent evolutionary species.
Keywords: C. Elegans; Fibroblasts; Glycosylation; Mitochondria; N-glycanase.
Copyright © 2017. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures
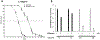
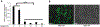


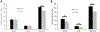
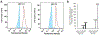
Similar articles
-
NGLY1 Deficiency, a Congenital Disorder of Deglycosylation: From Disease Gene Function to Pathophysiology.Cells. 2022 Mar 29;11(7):1155. doi: 10.3390/cells11071155. Cells. 2022. PMID: 35406718 Free PMC article. Review.
-
N-Glycanase 1 Transcriptionally Regulates Aquaporins Independent of Its Enzymatic Activity.Cell Rep. 2019 Dec 24;29(13):4620-4631.e4. doi: 10.1016/j.celrep.2019.11.097. Cell Rep. 2019. PMID: 31875565
-
N-glycanase NGLY1 regulates mitochondrial homeostasis and inflammation through NRF1.J Exp Med. 2018 Oct 1;215(10):2600-2616. doi: 10.1084/jem.20180783. Epub 2018 Aug 22. J Exp Med. 2018. PMID: 30135079 Free PMC article.
-
Drug screens of NGLY1 deficiency in worm and fly models reveal catecholamine, NRF2 and anti-inflammatory-pathway activation as potential clinical approaches.Dis Model Mech. 2019 Nov 4;12(11):dmm040576. doi: 10.1242/dmm.040576. Dis Model Mech. 2019. PMID: 31615832 Free PMC article.
-
Comprehensive Analysis of the Structure and Function of Peptide:N-Glycanase 1 and Relationship with Congenital Disorder of Deglycosylation.Nutrients. 2022 Apr 19;14(9):1690. doi: 10.3390/nu14091690. Nutrients. 2022. PMID: 35565658 Free PMC article. Review.
Cited by
-
Liver Involvement in Congenital Disorders of Glycosylation and Deglycosylation.Front Pediatr. 2021 Jul 5;9:696918. doi: 10.3389/fped.2021.696918. eCollection 2021. Front Pediatr. 2021. PMID: 34291020 Free PMC article.
-
Systemic gene therapy corrects the neurological phenotype in a mouse model of NGLY1 deficiency.JCI Insight. 2024 Oct 8;9(19):e183189. doi: 10.1172/jci.insight.183189. JCI Insight. 2024. PMID: 39137042 Free PMC article.
-
Ferroptosis regulation by the NGLY1/NFE2L1 pathway.Proc Natl Acad Sci U S A. 2022 Mar 15;119(11):e2118646119. doi: 10.1073/pnas.2118646119. Epub 2022 Mar 10. Proc Natl Acad Sci U S A. 2022. PMID: 35271393 Free PMC article.
-
NGLY1 Deficiency, a Congenital Disorder of Deglycosylation: From Disease Gene Function to Pathophysiology.Cells. 2022 Mar 29;11(7):1155. doi: 10.3390/cells11071155. Cells. 2022. PMID: 35406718 Free PMC article. Review.
-
A conserved role for AMP-activated protein kinase in NGLY1 deficiency.PLoS Genet. 2020 Dec 14;16(12):e1009258. doi: 10.1371/journal.pgen.1009258. eCollection 2020 Dec. PLoS Genet. 2020. PMID: 33315951 Free PMC article.
References
-
- Caglayan AO, Comu S, Baranoski JF, Parman Y, Kaymakcalan H, Akgumus GT, Caglar C, Dolen D, Erson-Omay EZ, Harmanci AS, Mishra-Gorur K, Freeze HH, Yasuno K, Bilguvar K, Gunel M, 2015. NGLY1 mutation causes neuromotor impairment, intellectual disability, and neuropathy. Eur. J. Med. Genet 58, 39–43. - PMC - PubMed
-
- Chantret I, Moore SE, 2008. Free oligosaccharide regulation during mammalian protein N-glycosylation. Glycobiology 18, 210–224. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

