Effect of Rifampin on the Single-Dose Pharmacokinetics of Oral Cabotegravir in Healthy Subjects
- PMID: 28739783
- PMCID: PMC5610536
- DOI: 10.1128/AAC.00487-17
Effect of Rifampin on the Single-Dose Pharmacokinetics of Oral Cabotegravir in Healthy Subjects
Abstract
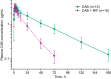
Drug-drug interactions between antiretroviral medications and rifampin complicate the treatment of HIV and tuberculosis coinfection. This study evaluated the effect of rifampin on the pharmacokinetics of oral cabotegravir, an integrase strand transfer inhibitor being investigated for long-acting treatment and prevention of HIV-1 infection. This was a phase I, single-center, open-label, fixed-sequence crossover study in healthy adults. The objective was to evaluate the effect of steady-state rifampin on the single-dose plasma pharmacokinetics of cabotegravir. Subjects received a single oral dose of cabotegravir (30 mg) on day 1 followed by plasma sampling on days 1 to 8. Treatment with once-daily oral rifampin (600 mg) occurred on days 8 to 28. Subjects received a second dose of 30 mg cabotegravir on day 21 followed by pharmacokinetic sampling on days 21 to 28. Fifteen subjects were enrolled and completed the study. Rifampin decreased the cabotegravir area under the concentration-time curve from 0 h to infinity and the half-life by 59% and 57%, respectively, whereas oral clearance was increased 2.4-fold. The maximum concentration of cabotegravir in plasma was unaffected by coadministration with rifampin. All adverse events were mild in severity, with chromaturia attributed to rifampin observed in all subjects. Rifampin induction of cabotegravir metabolism resulted in increased cabotegravir oral clearance and significantly decreased cabotegravir exposures. Rifampin is expected to increase cabotegravir clearance following long-acting injectable administration. Concomitant administration of rifampin with oral and long-acting formulations of cabotegravir is not recommended currently without further study. (This study has been registered at ClinicalTrials.gov under registration no. NCT02411435.).
Keywords: HIV-1; cabotegravir; drug-drug interactions; integrase strand transfer inhibitors; pharmacokinetics; rifampin.
Copyright © 2017 Ford et al.
Figures

Similar articles
-
Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial.Lancet HIV. 2017 Aug;4(8):e331-e340. doi: 10.1016/S2352-3018(17)30068-1. Epub 2017 May 22. Lancet HIV. 2017. PMID: 28546090 Clinical Trial.
-
Effect of rifabutin on the pharmacokinetics of oral cabotegravir in healthy subjects.Antivir Ther. 2019;24(4):301-308. doi: 10.3851/IMP3306. Antivir Ther. 2019. PMID: 30896438 Clinical Trial.
-
Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial.PLoS Med. 2018 Nov 8;15(11):e1002690. doi: 10.1371/journal.pmed.1002690. eCollection 2018 Nov. PLoS Med. 2018. PMID: 30408115 Free PMC article. Clinical Trial.
-
Pharmacokinetics and Pharmacodynamics of Cabotegravir, a Long-Acting HIV Integrase Strand Transfer Inhibitor.Eur J Drug Metab Pharmacokinet. 2019 Jun;44(3):319-327. doi: 10.1007/s13318-018-0526-2. Eur J Drug Metab Pharmacokinet. 2019. PMID: 30387005 Review.
-
Formulation and pharmacology of long-acting cabotegravir.Curr Opin HIV AIDS. 2015 Jul;10(4):239-45. doi: 10.1097/COH.0000000000000168. Curr Opin HIV AIDS. 2015. PMID: 26049948 Free PMC article. Review.
Cited by
-
Pharmacokinetics and Drug-Drug Interactions of Long-Acting Intramuscular Cabotegravir and Rilpivirine.Clin Pharmacokinet. 2021 Jul;60(7):835-853. doi: 10.1007/s40262-021-01005-1. Epub 2021 Apr 8. Clin Pharmacokinet. 2021. PMID: 33830459 Free PMC article. Review.
-
Evaluating cabotegravir/rilpivirine long-acting, injectable in the treatment of HIV infection: emerging data and therapeutic potential.HIV AIDS (Auckl). 2019 Jul 31;11:179-192. doi: 10.2147/HIV.S184642. eCollection 2019. HIV AIDS (Auckl). 2019. PMID: 31447590 Free PMC article.
-
Predicting Drug-Drug Interactions Between Rifampicin and Long-Acting Cabotegravir and Rilpivirine Using Physiologically Based Pharmacokinetic Modeling.J Infect Dis. 2019 May 5;219(11):1735-1742. doi: 10.1093/infdis/jiy726. J Infect Dis. 2019. PMID: 30566691 Free PMC article.
-
Drug-drug interactions between COVID-19 therapeutics and antiretroviral treatment: the evidence to date.Expert Opin Drug Metab Toxicol. 2023 Jul-Dec;19(11):795-806. doi: 10.1080/17425255.2023.2267970. Epub 2023 Nov 17. Expert Opin Drug Metab Toxicol. 2023. PMID: 37800561 Free PMC article. Review.
-
Predicting Drug-Drug Interactions Involving Rifampicin Using a Semi-mechanistic Hepatic Compartmental Model.Pharm Res. 2024 Apr;41(4):699-709. doi: 10.1007/s11095-024-03691-5. Epub 2024 Mar 22. Pharm Res. 2024. PMID: 38519815
References
-
- World Health Organization. October 2016. World Health Organization tuberculosis fact sheet 104. http://www.who.int/mediacentre/factsheets/fs104/en Accessed 22 June 2017.
-
- Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, Madec Y, Marcy O, Chan S, Prak N, Kim C, Lak KK, Hak C, Dim B, Sin CI, Sun S, Guillard B, Sar B, Vong S, Fernandez M, Fox L, Delfraissy JF, Goldfeld AE, CAMELIA (ANRS 1295–CIPRA KH001) Study Team. 2011. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 365:1471–1481. doi:10.1056/NEJMoa1013911. - DOI - PMC - PubMed
-
- World Health Organization. January 2006. World Health Organization international standards for tuberculosis care. http://www.who.int/tb/publications/2006/istc_report.pdf Accessed 22 June 2017.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

